An important role of the SRC family kinase Lyn in stimulating platelet granule secretion
- PMID: 20189992
- PMCID: PMC2857081
- DOI: 10.1074/jbc.M109.098756
An important role of the SRC family kinase Lyn in stimulating platelet granule secretion
Abstract
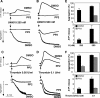
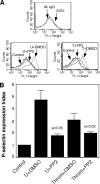
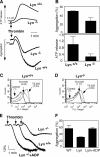
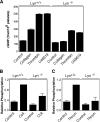
The Src family kinases (SFKs) have been proposed to play stimulatory and inhibitory roles in platelet activation. The mechanisms for these apparently contradictory roles are unclear. Here we show that SFK, mainly Lyn, is important in stimulating a common signaling pathway leading to secretion of platelet granules. Lyn knock-out or an isoform-nonselective SFK inhibitor, PP2, inhibited platelet secretion of both dense and alpha granules and the secretion-dependent platelet aggregation induced by thrombin, collagen, and thromboxane A(2). The inhibitory effect of Lyn knock-out on platelet aggregation was reversed by supplementing granule content ADP, indicating that the primary role of Lyn is to stimulate granule secretion. Inhibitory effect of PP2 on platelet aggregation induced by thrombin and thromboxane A(2) were also reversed by supplementing ADP. Furthermore, PP2 treatment or Lyn knock-out diminished agonist-induced Akt activation and cyclic GMP production. The inhibitory effect of PP2 or Lyn knock-out on platelet response can be corrected by supplementing cyclic GMP. These data indicate that Lyn stimulates platelet secretion by activating the phosphoinositide 3-kinase-Akt-nitric oxide (NO)-cyclic GMP pathway and also provide an explanation why Lyn can both stimulate and inhibit platelet activation.
Figures






Similar articles
-
The role of Rac1 in glycoprotein Ib-IX-mediated signal transduction and integrin activation.Arterioscler Thromb Vasc Biol. 2012 Nov;32(11):2761-8. doi: 10.1161/ATVBAHA.112.254920. Epub 2012 Sep 20. Arterioscler Thromb Vasc Biol. 2012. PMID: 22995516 Free PMC article.
-
Role of the Src family kinase Lyn in TxA2 production, adenosine diphosphate secretion, Akt phosphorylation, and irreversible aggregation in platelets stimulated with gamma-thrombin.Blood. 2002 Apr 1;99(7):2442-7. doi: 10.1182/blood.v99.7.2442. Blood. 2002. PMID: 11895777
-
Regulation of platelet guanylyl cyclase by collagen: evidence that Glycoprotein VI mediates platelet nitric oxide synthesis in response to collagen.Thromb Haemost. 2005 Aug;94(2):395-403. doi: 10.1160/TH05-01-0027. Thromb Haemost. 2005. PMID: 16113831
-
Two waves of platelet secretion induced by thromboxane A2 receptor and a critical role for phosphoinositide 3-kinases.J Biol Chem. 2003 Aug 15;278(33):30725-31. doi: 10.1074/jbc.M301838200. Epub 2003 Jun 9. J Biol Chem. 2003. PMID: 12796499
-
[Activators, receptors and signal transduction pathways of blood platelets].Biomed Khim. 2014 Mar-Apr;60(2):182-200. doi: 10.18097/pbmc20146002182. Biomed Khim. 2014. PMID: 24837309 Review. Russian.
Cited by
-
Kindlin-2 regulates hemostasis by controlling endothelial cell-surface expression of ADP/AMP catabolic enzymes via a clathrin-dependent mechanism.Blood. 2013 Oct 3;122(14):2491-9. doi: 10.1182/blood-2013-04-497669. Epub 2013 Jul 29. Blood. 2013. PMID: 23896409 Free PMC article.
-
14-3-3 proteins in platelet biology and glycoprotein Ib-IX signaling.Blood. 2018 May 31;131(22):2436-2448. doi: 10.1182/blood-2017-09-742650. Epub 2018 Apr 5. Blood. 2018. PMID: 29622550 Free PMC article. Review.
-
Signaling during platelet adhesion and activation.Arterioscler Thromb Vasc Biol. 2010 Dec;30(12):2341-9. doi: 10.1161/ATVBAHA.110.207522. Epub 2010 Nov 11. Arterioscler Thromb Vasc Biol. 2010. PMID: 21071698 Free PMC article. Review.
-
The role of Rac1 in glycoprotein Ib-IX-mediated signal transduction and integrin activation.Arterioscler Thromb Vasc Biol. 2012 Nov;32(11):2761-8. doi: 10.1161/ATVBAHA.112.254920. Epub 2012 Sep 20. Arterioscler Thromb Vasc Biol. 2012. PMID: 22995516 Free PMC article.
-
Maintenance of murine platelet homeostasis by the kinase Csk and phosphatase CD148.Blood. 2018 Mar 8;131(10):1122-1144. doi: 10.1182/blood-2017-02-768077. Epub 2018 Jan 4. Blood. 2018. PMID: 29301754 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

