Characterization of Acp, a peptidoglycan hydrolase of Clostridium perfringens with N-acetylglucosaminidase activity that is implicated in cell separation and stress-induced autolysis
- PMID: 20190047
- PMCID: PMC2863477
- DOI: 10.1128/JB.01546-09
Characterization of Acp, a peptidoglycan hydrolase of Clostridium perfringens with N-acetylglucosaminidase activity that is implicated in cell separation and stress-induced autolysis
Abstract
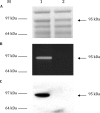
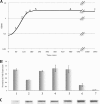
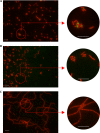
This work reports the characterization of the first known peptidoglycan hydrolase (Acp) produced mainly during vegetative growth of Clostridium perfringens. Acp has a modular structure with three domains: a signal peptide domain, an N-terminal domain with repeated sequences, and a C-terminal catalytic domain. The purified recombinant catalytic domain of Acp displayed lytic activity on the cell walls of several Gram-positive bacterial species. Its hydrolytic specificity was established by analyzing the Bacillus subtilis peptidoglycan digestion products by coupling reverse phase-high-pressure liquid chromatography (RP-HPLC) and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) analysis, which displayed an N-acetylglucosaminidase activity. The study of acp expression showed a constant expression during growth, which suggested an important role of Acp in growth of C. perfringens. Furthermore, cell fractionation and indirect immunofluorescence staining using anti-Acp antibodies revealed that Acp is located at the septal peptidoglycan of vegetative cells during exponential growth phase, indicating a role in cell separation or division of C. perfringens. A knockout acp mutant strain was obtained by using the insertion of mobile group II intron strategy (ClosTron). The microscopic examination indicated a lack of vegetative cell separation in the acp mutant strain, as well as the wild-type strain incubated with anti-Acp antibodies, demonstrating the critical role of Acp in cell separation. The comparative responses of wild-type and acp mutant strains to stresses induced by Triton X-100, bile salts, and vancomycin revealed an implication of Acp in autolysis induced by these stresses. Overall, Acp appears as a major cell wall N-acetylglucosaminidase implicated in both vegetative growth and stress-induced autolysis.
Figures








References
-
- Allignet, J., P. England, I. Old, and N. El Solh. 2002. Several regions of the repeat domain of the Staphylococcus caprae autolysin, AtlC, are involved in fibronectin binding. FEMS Microbiol. Lett. 213:193-197. - PubMed
-
- Bateman, A., and M. Bycroft. 2000. The structure of a LysM domain from E. coli membrane-bound lytic murein transglycosylase D (MltD). J. Mol. Biol. 299:1113-1119. - PubMed
-
- Bourgeois, I., E. Camiade, R. Biswas, P. Courtin, L. Gibert, F. Gotz, M. P. Chapot-Chartier, J. L. Pons, and M. Pestel-Caron. 2009. Characterization of AtlL, a bifunctional autolysin of Staphylococcus lugdunensis with N-acetylglucosaminidase and N-acetylmuramoyl-l-alanine amidase activities. FEMS Microbiol. Lett. 290:105-113. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

