Major role for cysteine proteases during the early phase of Acanthamoeba castellanii encystment
- PMID: 20190073
- PMCID: PMC2863413
- DOI: 10.1128/EC.00300-09
Major role for cysteine proteases during the early phase of Acanthamoeba castellanii encystment
Abstract
Acanthamoeba castellanii is a facultative pathogen that has a two-stage life cycle comprising the vegetatively growing trophozoite stage and the dormant cyst stage. Cysts are formed when the cell encounters unfavorable conditions, such as environmental stress or food deprivation. Due to their rigid double-layered wall, Acanthamoeba cysts are highly resistant to antiamoebic drugs. This is problematic as cysts can survive initially successful chemotherapeutic treatment and cause relapse of the disease. We studied the Acanthamoeba encystment process by using two-dimensional gel electrophoresis (2DE) and found that most changes in the protein content occur early in the process. Truncated actin isoforms were found to abound in the encysting cell, and the levels of translation elongation factor 2 (EF2) were sharply decreased, indicating that the rate of protein synthesis must be low at this stage. In the advanced stage of encystment, however, EF2 levels and the trophozoite proteome were partly restored. The protease inhibitors PMSF (phenylmethylsulfonyl fluoride) and E64d [(2S,3S)-trans-epoxysuccinyl-L-leucylamido-3-methylbutane ethyl ester] inhibited the onset of encystment, whereas the protein synthesis inhibitor cycloheximide was ineffective. Changes in the protein profile, similar to those of encysting cells, could be observed with trophozoite homogenates incubated at room temperature for several hours. Interestingly, these changes could be inhibited significantly by cysteine protease inhibitors but not by inhibitors against other proteases. Taken together, we conclude that the encystment process in A. castellanii is of a bipartite nature consisting of an initial phase of autolysis and protein degradation and an advanced stage of restoration accompanied by the expression of encystment-specific genes.
Figures
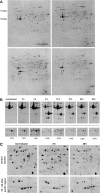
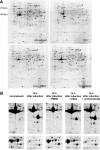
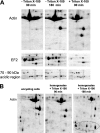

References
-
- Bouyer S., Rodier M. H., Guillot A., Héchard Y. 2009. Acanthamoeba castellanii: proteins involved in actin dynamics, glycolysis, and proteolysis are regulated during encystation. Exp. Parasitol. 123:90–94 - PubMed
-
- Chen L., Orfeo T., Gilmartin G., Bateman E. 2004. Mechanism of cyst specific protein 21 mRNA induction during Acanthamoeba differentiation. Biochim. Biophys. Acta 1691:23–31 - PubMed
-
- De Jonckheere J. F. 1991. Ecology of Acanthamoeba. Rev. Infect. Dis. 13(Suppl. 5):S385–S387 - PubMed
-
- Dudley R., Alsam S., Khan N. A. 2008. The role of proteases in the differentiation of Acanthamoeba castellanii. FEMS Microbiol. Lett. 286:9–15 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

