Heterogeneous ventricular sympathetic innervation, altered beta-adrenergic receptor expression, and rhythm instability in mice lacking the p75 neurotrophin receptor
- PMID: 20190098
- PMCID: PMC2886645
- DOI: 10.1152/ajpheart.01128.2009
Heterogeneous ventricular sympathetic innervation, altered beta-adrenergic receptor expression, and rhythm instability in mice lacking the p75 neurotrophin receptor
Abstract
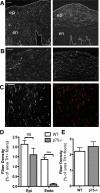
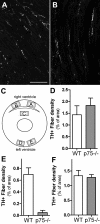
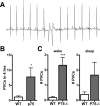
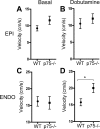
Sympathetic nerves stimulate cardiac function through the release of norepinephrine and the activation of cardiac beta(1)-adrenergic receptors. The sympathetic innervation of the heart is sculpted during development by chemoattractive factors including nerve growth factor (NGF) and the chemorepulsive factor semaphorin 3a. NGF acts through the TrkA receptor and the p75 neurotrophin receptor (p75(NTR)) in sympathetic neurons. NGF stimulates sympathetic axon extension into the heart through TrkA, but p75(NTR) modulates multiple coreceptors that can either stimulate or inhibit axon outgrowth. In mice lacking p75(NTR), the sympathetic innervation density in target tissues ranges from denervation to hyperinnervation. Recent studies have revealed significant changes in the sympathetic innervation density of p75NTR-deficient (p75(NTR-/-)) atria between early postnatal development and adulthood. We examined the innervation of adult p75(NTR-/-) ventricles and discovered that the subendocardium of the p75(NTR-/-) left ventricle was essentially devoid of sympathetic nerve fibers, whereas the innervation density of the subepicardium was normal. This phenotype is similar to that seen in mice overexpressing semaphorin 3a, and we found that sympathetic axons lacking p75(NTR) are more sensitive to semaphorin 3a in vitro than control neurons. The lack of subendocardial innervation was associated with decreased dP/dt, altered cardiac beta(1)-adrenergic receptor expression and sensitivity, and a significant increase in spontaneous ventricular arrhythmias. The lack of p75(NTR) also resulted in increased tyrosine hydroxylase content in cardiac sympathetic neurons and elevated norepinephrine in the right ventricle, where innervation density was normal.
Figures




Comment in
-
The p75 neurotrophin receptor, semaphorins, and sympathetic traffic in the heart.Am J Physiol Heart Circ Physiol. 2010 Jun;298(6):H1633-6. doi: 10.1152/ajpheart.00253.2010. Epub 2010 Mar 19. Am J Physiol Heart Circ Physiol. 2010. PMID: 20304820 Free PMC article. No abstract available.
References
-
- Aronow WS, Ahn C, Mercando AD, Epstein S. Circadian variation of sudden cardiac death or fatal myocardial infarction is abolished by propranolol in patients with heart disease and complex ventricular arrhythmias. Am J Cardiol 74: 819–821, 1994 - PubMed
-
- Bandtlow C, Dechant G. From cell death to neuronal regeneration, effects of the p75 neurotrophin receptor depend on interactions with partner subunits. Sci STKE 2004: pe24, 2004 - PubMed
-
- Bjerre B, Bjorklund A, Mobley W, Rosengren E. Short- and long-term effects of nerve growth factor on the sympathetic nervous system in the adult mouse. Brain Res 94: 263–277, 1975 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

