MHC class I molecules with Superenhanced CD8 binding properties bypass the requirement for cognate TCR recognition and nonspecifically activate CTLs
- PMID: 20190139
- PMCID: PMC3024536
- DOI: 10.4049/jimmunol.0902398
MHC class I molecules with Superenhanced CD8 binding properties bypass the requirement for cognate TCR recognition and nonspecifically activate CTLs
Abstract
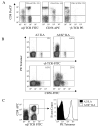
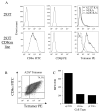
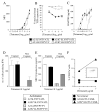
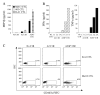
CD8(+) CTLs are essential for effective immune defense against intracellular microbes and neoplasia. CTLs recognize short peptide fragments presented in association with MHC class I (MHCI) molecules on the surface of infected or dysregulated cells. Ag recognition involves the binding of both TCR and CD8 coreceptor to a single ligand (peptide MHCI [pMHCI]). The TCR/pMHCI interaction confers Ag specificity, whereas the pMHCI/CD8 interaction mediates enhanced sensitivity to Ag. Striking biophysical differences exist between the TCR/pMHCI and pMHCI/CD8 interactions; indeed, the pMHCI/CD8 interaction can be >100-fold weaker than the cognate TCR/pMHCI interaction. In this study, we show that increasing the strength of the pMHCI/CD8 interaction by approximately 15-fold results in nonspecific, cognate Ag-independent pMHCI tetramer binding at the cell surface. Furthermore, pMHCI molecules with superenhanced affinity for CD8 activate CTLs in the absence of a specific TCR/pMHCI interaction to elicit a full range of effector functions, including cytokine/chemokine release, degranulation and proliferation. Thus, the low solution binding affinity of the pMHCI/CD8 interaction is essential for the maintenance of CTL Ag specificity.
Figures

Similar articles
-
Different T cell receptor affinity thresholds and CD8 coreceptor dependence govern cytotoxic T lymphocyte activation and tetramer binding properties.J Biol Chem. 2007 Aug 17;282(33):23799-810. doi: 10.1074/jbc.M700976200. Epub 2007 May 31. J Biol Chem. 2007. PMID: 17540778
-
Anti-CD8 antibodies can inhibit or enhance peptide-MHC class I (pMHCI) multimer binding: this is paralleled by their effects on CTL activation and occurs in the absence of an interaction between pMHCI and CD8 on the cell surface.J Immunol. 2003 Dec 15;171(12):6650-60. doi: 10.4049/jimmunol.171.12.6650. J Immunol. 2003. PMID: 14662868
-
Anti-CD8 antibodies can trigger CD8+ T cell effector function in the absence of TCR engagement and improve peptide-MHCI tetramer staining.J Immunol. 2011 Jul 15;187(2):654-63. doi: 10.4049/jimmunol.1003941. Epub 2011 Jun 15. J Immunol. 2011. PMID: 21677135 Free PMC article.
-
The multiple roles of the CD8 coreceptor in T cell biology: opportunities for the selective modulation of self-reactive cytotoxic T cells.J Leukoc Biol. 2011 Dec;90(6):1089-99. doi: 10.1189/jlb.0611316. Epub 2011 Sep 27. J Leukoc Biol. 2011. PMID: 21954283 Review.
-
Molecular mechanisms and biological significance of CTL avidity.Curr HIV Res. 2003 Jul;1(3):287-94. doi: 10.2174/1570162033485230. Curr HIV Res. 2003. PMID: 15046253 Review.
Cited by
-
CD8 controls T cell cross-reactivity.J Immunol. 2010 Oct 15;185(8):4625-32. doi: 10.4049/jimmunol.1001480. Epub 2010 Sep 15. J Immunol. 2010. PMID: 20844204 Free PMC article.
-
Using X-ray Crystallography, Biophysics, and Functional Assays to Determine the Mechanisms Governing T-cell Receptor Recognition of Cancer Antigens.J Vis Exp. 2017 Feb 6;(120):54991. doi: 10.3791/54991. J Vis Exp. 2017. PMID: 28287509 Free PMC article.
-
Proximity of TCR and its CD8 coreceptor controls sensitivity of T cells.Immunol Lett. 2014 Jan-Feb;157(1-2):16-22. doi: 10.1016/j.imlet.2013.11.005. Epub 2013 Nov 18. Immunol Lett. 2014. PMID: 24263053 Free PMC article.
-
The structural basis of chicken, swine and bovine CD8αα dimers provides insight into the co-evolution with MHC I in endotherm species.Sci Rep. 2016 Apr 28;6:24788. doi: 10.1038/srep24788. Sci Rep. 2016. PMID: 27122108 Free PMC article.
-
The molecular determinants of CD8 co-receptor function.Immunology. 2012 Oct;137(2):139-48. doi: 10.1111/j.1365-2567.2012.03625.x. Immunology. 2012. PMID: 22804746 Free PMC article. Review.
References
-
- Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–544. - PubMed
-
- Rudolph MG, Wilson IA. The specificity of TCR/pMHC interaction. Curr Opin Immunol. 2002;14:52–65. - PubMed
-
- Zamoyska R. CD4 and CD8: modulators of T-cell receptor recognition of antigen and of immune responses? Curr Opin Immunol. 1998;10:82–87. - PubMed
-
- Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 2003;18:255–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

