The safety and efficacy of intravenous ferric carboxymaltose in anaemic patients undergoing haemodialysis: a multi-centre, open-label, clinical study
- PMID: 20190247
- PMCID: PMC2905444
- DOI: 10.1093/ndt/gfq069
The safety and efficacy of intravenous ferric carboxymaltose in anaemic patients undergoing haemodialysis: a multi-centre, open-label, clinical study
Abstract
Background: Patients with chronic kidney disease (CKD) often present with iron depletion and iron deficiency anaemia (IDA) because of frequent blood (and iron) loss. Therapy consists of repletion of iron stores and intravenous (i.v.) iron has become the standard care in this setting. However, older i.v. iron preparations have their limitations. This study primarily investigated the safety, and also the efficacy, of ferric carboxymaltose (FCM), a next-generation i.v. iron formulation, given as a bolus-push injection in patients with CKD undergoing maintenance haemodialysis (HD).
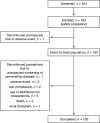
Methods: Patients (aged 18-65 years) with IDA undergoing HD received 100-200 mg of iron as FCM via an i.v. bolus-push injection into the HD venous line, two to three times weekly for <or=6 weeks. Safety assessments included incidence of adverse events (AEs). Treatment responders were patients attaining >or=1.0 g/dl increase in haemoglobin (Hb) from baseline at any time during the study. Enrolled patients (safety population) receiving >or=1 dose of study medication were included in the efficacy analyses [intent-to-treat (ITT) population].
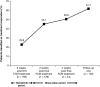
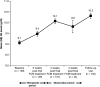
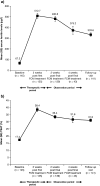
Results: Of 163 patients enrolled, 150 (92%) completed the study. The mean +/- SD total cumulative dose of iron as FCM administered was 2133.3 +/- 57.7 mg. In total, 193 AEs were reported in 89 out of 163 (54.6%) patients. Almost three-quarters of patients (73.6%) received erythropoiesis-stimulating agents (ESAs), but the dose remained stable during the study. Serious AEs occurred in 12 out of 163 (7.4%) patients and two patients died; none of these was considered by the investigator to be related to the study medication. Only five out of 163 (3.1%) patients discontinued study medication due to an AE. Overall, 100 out of 162 (61.7%; ITT population) patients were treatment responders, and mean Hb levels increased from 9.1 +/- 1.30 g/dl at baseline to 10.3 +/- 1.63 g/dl at follow-up.
Conclusions: FCM is well-tolerated and effective in the correction of Hb levels and iron stores in patients with IDA undergoing HD. As changes in anaemia treatment other than i.v. FCM (e.g. increased ESA doses) were not permitted during the study, the clinically relevant increase in Hb in the majority of patients can be solely attributed to efficient iron utilization. The incidence of AEs was as expected for this population.
Figures
Similar articles
-
Pharmacodynamics and safety of ferric carboxymaltose: a multiple-dose study in patients with iron-deficiency anaemia secondary to a gastrointestinal disorder.Arzneimittelforschung. 2010;60(6a):373-85. doi: 10.1055/s-0031-1296302. Arzneimittelforschung. 2010. PMID: 20648929 Clinical Trial.
-
Pharmacokinetics, safety and tolerability of intravenous ferric carboxymaltose: a dose-escalation study in volunteers with mild iron-deficiency anaemia.Arzneimittelforschung. 2010;60(6a):362-72. doi: 10.1055/s-0031-1296301. Arzneimittelforschung. 2010. PMID: 20648928 Clinical Trial.
-
TIDILAP: Treatment of iron deficiency in lipoprotein apheresis patients --A prospective observational multi-center cohort study comparing efficacy, safety and tolerability of ferric gluconate with ferric carboxymaltose.Atheroscler Suppl. 2015 May;18:199-208. doi: 10.1016/j.atherosclerosissup.2015.02.030. Atheroscler Suppl. 2015. PMID: 25936327
-
Ferric carboxymaltose: a review of its use in iron-deficiency anaemia.Drugs. 2009;69(6):739-56. doi: 10.2165/00003495-200969060-00007. Drugs. 2009. PMID: 19405553 Review.
-
Comparative efficacy and safety of intravenous ferric carboxymaltose and iron sucrose for iron deficiency anemia in obstetric and gynecologic patients: A systematic review and meta-analysis.Medicine (Baltimore). 2021 May 21;100(20):e24571. doi: 10.1097/MD.0000000000024571. Medicine (Baltimore). 2021. PMID: 34011020 Free PMC article.
Cited by
-
Efficacy, Safety and Pharmacoeconomic Analysis of Intravenous Ferric Carboxymaltose in Anemic Hemodialysis Patients Unresponsive to Ferric Gluconate Treatment: A Multicenter Retrospective Study.J Clin Med. 2022 Sep 7;11(18):5284. doi: 10.3390/jcm11185284. J Clin Med. 2022. PMID: 36142929 Free PMC article.
-
Intravenous iron therapy in patients with chronic kidney disease: recent evidence and future directions.Clin Kidney J. 2017 Dec;10(Suppl 1):i16-i24. doi: 10.1093/ckj/sfx043. Epub 2017 Nov 28. Clin Kidney J. 2017. PMID: 29225819 Free PMC article. Review.
-
REGAIN STUDY: Retrospective Study to Assess the Effectiveness, Tolerability, and Safety of Ferric Carboxymaltose in the Management of Iron Deficiency Anemia in Pregnant Women.Anemia. 2019 Nov 12;2019:4640635. doi: 10.1155/2019/4640635. eCollection 2019. Anemia. 2019. PMID: 31781389 Free PMC article.
-
Economic Evaluation of Ferric Carboxymaltose for the Management of Hemodialysis Patients with Iron Deficiency Anemia in Italy.Adv Ther. 2019 Nov;36(11):3253-3264. doi: 10.1007/s12325-019-01089-z. Epub 2019 Sep 5. Adv Ther. 2019. PMID: 31489572 Free PMC article.
-
The Switch from Ferric Gluconate to Ferric Carboxymaltose in Hemodialysis Patients Acts on Iron Metabolism, Erythropoietin, and Costs: A Retrospective Analysis.Medicina (Kaunas). 2023 Jun 2;59(6):1071. doi: 10.3390/medicina59061071. Medicina (Kaunas). 2023. PMID: 37374275 Free PMC article.
References
-
- Locatelli F, Aljama P, Barany P, et al. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004;19:ii1–47. - PubMed
-
- Hörl WH. Iron therapy in patients with chronic kidney disease: taking the high road? Port J Nephrol Hypert. 2009;23:5–10.
-
- Royal College of Physicians (London). National Collaborating Centre for Chronic Conditions Anaemia management in chronic kidney disease: national clinical guideline for management in adults and children. 2006. Available from: http://www.nice.org.uk/ - PubMed
-
- Funk F, Ryle P, Canclini C, Neiser S, Geisser P. The new generation of intravenous iron: chemistry, pharmacology and toxicology of ferric carboxymaltose. Arzneimittelforschung. 2010 Supplement: In press. - PubMed
-
- World Health Organization . Iron Deficiency Anemia: Assessment, Prevention and Control. Report of a Joint WHO/UNICEF/UNU Consultation. 1998.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

