Drf1-dependent kinase interacts with Claspin through a conserved protein motif
- PMID: 20190277
- PMCID: PMC2857117
- DOI: 10.1074/jbc.M109.077370
Drf1-dependent kinase interacts with Claspin through a conserved protein motif
Abstract
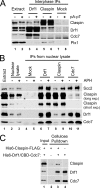
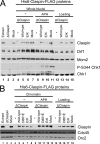
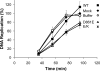
The Dbf4/Drf1-dependent kinase (DDK) is required for the initiation of DNA replication in eukaryotes. Another protein, Claspin, mediates the activation of a cellular checkpoint response to stalled replication forks and is also a regulator of replication. In this study, we found that DDK phosphorylates Claspin in vitro and forms a nuclear complex containing Cdc7, Drf1, and Claspin in Xenopus egg extracts. In addition, purified Claspin and DDK are capable of a direct in vitro interaction. We identified a conserved binding site on Claspin required for its interaction with DDK. This site corresponds to the first of two sequence repeats in the Chk1-binding domain of Claspin. Furthermore, we have established that two amino acids in this motif, Asp(861) and Gln(866), are essential for the interaction between Claspin and DDK. We found that mutant forms of Claspin incapable of interacting with DDK are still able to associate with and activate Chk1 in response to DNA replication blockages. However, Claspin-depleted egg extracts that have been reconstituted with these mutants of Claspin undergo DNA replication more slowly. These findings suggest that the interaction of DDK with Claspin mediates a checkpoint-independent function of Claspin related to DNA replication.
Figures



Similar articles
-
The role of Dbf4/Drf1-dependent kinase Cdc7 in DNA-damage checkpoint control.Mol Cell. 2008 Dec 26;32(6):862-9. doi: 10.1016/j.molcel.2008.12.005. Mol Cell. 2008. PMID: 19111665 Free PMC article.
-
Cdc7-Drf1 is a developmentally regulated protein kinase required for the initiation of vertebrate DNA replication.Genes Dev. 2005 Oct 1;19(19):2295-300. doi: 10.1101/gad.1339805. Genes Dev. 2005. PMID: 16204181 Free PMC article.
-
Xenopus CDC7/DRF1 complex is required for the initiation of DNA replication.J Biol Chem. 2006 Apr 28;281(17):11569-76. doi: 10.1074/jbc.M510278200. Epub 2006 Feb 28. J Biol Chem. 2006. PMID: 16507577
-
Claspin - checkpoint adaptor and DNA replication factor.FEBS J. 2019 Feb;286(3):441-455. doi: 10.1111/febs.14594. Epub 2018 Jun 29. FEBS J. 2019. PMID: 29931808 Review.
-
Mrc1/Claspin: a new role for regulation of origin firing.Curr Genet. 2017 Oct;63(5):813-818. doi: 10.1007/s00294-017-0690-y. Epub 2017 Mar 29. Curr Genet. 2017. PMID: 28357499 Review.
Cited by
-
The Protective Role of Dormant Origins in Response to Replicative Stress.Int J Mol Sci. 2018 Nov 12;19(11):3569. doi: 10.3390/ijms19113569. Int J Mol Sci. 2018. PMID: 30424570 Free PMC article. Review.
-
Claspin recruits Cdc7 kinase for initiation of DNA replication in human cells.Nat Commun. 2016 Jul 12;7:12135. doi: 10.1038/ncomms12135. Nat Commun. 2016. PMID: 27401717 Free PMC article.
-
Cdc7 activates replication checkpoint by phosphorylating the Chk1-binding domain of Claspin in human cells.Elife. 2019 Dec 31;8:e50796. doi: 10.7554/eLife.50796. Elife. 2019. PMID: 31889509 Free PMC article.
-
Mrc1 marks early-firing origins and coordinates timing and efficiency of initiation in fission yeast.Mol Cell Biol. 2011 Jun;31(12):2380-91. doi: 10.1128/MCB.01239-10. Epub 2011 Apr 25. Mol Cell Biol. 2011. PMID: 21518960 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

