Rapid 3D phenotyping of cardiovascular development in mouse embryos by micro-CT with iodine staining
- PMID: 20190279
- PMCID: PMC3059892
- DOI: 10.1161/CIRCIMAGING.109.918482
Rapid 3D phenotyping of cardiovascular development in mouse embryos by micro-CT with iodine staining
Abstract
Background: Microcomputed tomography (micro-CT) has been used extensively in research to generate high-resolution 3D images of calcified tissues in small animals nondestructively. It has been especially useful for the characterization of skeletal mutations but limited in its utility for the analysis of soft tissue such as the cardiovascular system. Visualization of the cardiovascular system has been largely restricted to structures that can be filled with radiopaque intravascular contrast agents in adult animals. Recent ex vivo studies using osmium tetroxide, iodinated contrast agents, inorganic iodine, and phosphotungstic acid have demonstrated the ability to stain soft tissues differentially, allowing for high intertissue contrast in micro-CT images. In the present study, we demonstrate the application of this technology for visualization of cardiovascular structures in developing mouse embryos using Lugol solution (aqueous potassium iodide plus iodine).
Methods and results: We show the optimization of this method to obtain ex vivo micro-CT images of embryonic and neonatal mice with excellent soft-tissue contrast. We demonstrate the utility of this method to visualize key structures during cardiovascular development at various stages of embryogenesis. Our method benefits from the ease of sample preparation, low toxicity, and low cost. Furthermore, we show how multiple cardiac defects can be demonstrated by micro-CT in a single specimen with a known genetic lesion. Indeed, a previously undescribed cardiac venous abnormality is revealed in a PlexinD1 mutant mouse.
Conclusions: Micro-CT of iodine-stained tissue is a valuable technique for the characterization of cardiovascular development and defects in mouse models of congenital heart disease.
Conflict of interest statement
Figures
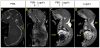
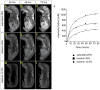
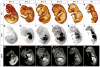



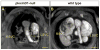
Comment in
-
Mouse models of congenital heart defects: what's missing?Circ Cardiovasc Imaging. 2010 May;3(3):228-30. doi: 10.1161/CIRCIMAGING.110.956979. Circ Cardiovasc Imaging. 2010. PMID: 20484112 Free PMC article. No abstract available.
References
-
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. - PubMed
-
- Horsthuis T, Christoffels VM, Anderson RH, Moorman AF. Can recent insights into cardiac development improve our understanding of congenitally malformed hearts? Clin Anat. 2009;22:4–20. - PubMed
-
- Soufan AT, van den Berg G, Moerland PD, Massink MM, van den Hoff MJ, Moorman AF, Ruijter JM. Three-dimensional measurement and visualization of morphogenesis applied to cardiac embryology. J Microsc. 2007;225:269–274. - PubMed
-
- Dhanantwari P, Lee E, Krishnan A, Samtani R, Yamada S, Anderson S, Lockett E, Dono-frio M, Shiota K, Leatherbury L, Lo CW. Human cardiac development in the first trimester: a high-resolution magnetic resonance imaging and episcopic fluorescence image capture atlas. Circulation. 2009;120:343–351. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

