The development and morphogenesis of the tendon-to-bone insertion - what development can teach us about healing -
- PMID: 20190378
- PMCID: PMC3605736
The development and morphogenesis of the tendon-to-bone insertion - what development can teach us about healing -
Abstract
The attachment of dissimilar materials is a major challenge because of the high levels of stress that develop at such interfaces. An effective solution to this problem develops at the attachment of tendon (a compliant "soft tissue") to bone (a stiff "hard tissue"). This tissue, the "enthesis", transitions from tendon to bone through gradations in structure, composition, and mechanical properties. These gradations are not regenerated during tendon-to-bone healing, leading to a high incidence of failure after surgical repair. Understanding the development of the enthesis may allow scientists to develop treatments that regenerate the natural tendon-to-bone insertion. Recent work has demonstrated that both biologic and mechanical factors drive the development and morphogenesis of the enthesis. A cascade of biologic signals similar to those seen in the growth plate promotes mineralization of cartilage on the bony end of the enthesis and the formation of fibrocartilage on the tendon end of the enthesis. Mechanical loading is also necessary for the development of the enthesis. Removal of muscle load impairs the formation of bone, fibrocartilage, and tendon at the developing enthesis. This paper reviews recent work on the development of the enthesis, with an emphasis on the roles of biologic and mechanical factors.
Figures
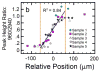
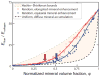
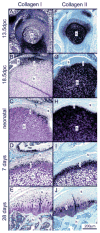

References
-
- Bostrom MPG, Boskey A, Kauffman JK, Einhorn TA. Form and function of bone. In: Buckwalter JA, Einhorn TA, Simon SR, editors. Orthopaedic Basic Science. 2. AAOS; 2000. pp. 319–70.
-
- Woo SL, An K, Frank CB, Livesay GA, Ma CB, Zeminski JA, Wayne JS, Myers BS. Orthopaedic Basic Science. 2. Rosemont, IL: AAOS; 2000. Anatomy, biology, and biomechanics of tendon and ligament; pp. 581–616.
-
- Jones RM. Mechanics of composite materials. 2. Philadelphia: Taylor & Francis, Inc; 1999.
-
- Williams ML. Stress singularities resulting from various boundary conditions in angular corners of plates in extension. Journal of Applied Mechanics. 1952;19:526–8.
-
- Aoki M, Oguma H, Fukushima S, Ishii S, Ohtani S, Murakami G. Fibrous connection to bone after immediate repair of the canine infraspinatus: the most effective bony surface for tendon attachment.[see comment] Journal of Shoulder & Elbow Surgery. 2001;10(2):123–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
