Role of muscle-derived growth factors in bone formation
- PMID: 20190381
- PMCID: PMC3753580
Role of muscle-derived growth factors in bone formation
Abstract
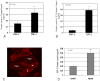
Muscle and bone anabolism and catabolism are tightly coupled during growth, development, and aging, yet the cellular and molecular mechanisms linking these two tissues are not well understood. Here we show that FGF-2 and IGF-1, two growth factors known to play a major role in regulating bone formation, are localized to muscle fibers along the muscle-bone interface of the mouse forelimb. Likewise, receptors for these growth factors are also abundant in periosteum adjacent to fleshy muscle attachments along the diaphysis of long bones. Growth factor levels were quantified from homogenized mouse forelimb muscles and IGF-1 was found to be the most abundant factor with FGF-2 also detected. Growth factor levels were also analyzed in conditioned medium from cultured myotubes, and IGF-1 and FGF-2 were again detected at significant levels. Mechanically wounding C2C12 myotubes increased the release of FGF-2 into conditioned medium, whereas IGF-1 was secreted at lower concentrations than FGF-2 following injury. Together these findings suggest that muscle is an important, local source of growth factors for bone tissue. Hence, the integrated growth and development of bone and muscle is likely to be regulated in part by paracrine mechanisms at the muscle-bone interface involving growth factor signaling.
Figures


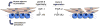
References
-
- Rauch F, Bailey D, Baxter-Jones A, Mirwald R, Faulkner R. The ‘muscle-bone unit’ during the pubertal growth spurt. Bone. 2004;34:771–775. - PubMed
-
- Greenlund LJ, Nair KS. Sarcopenia--consequences, mechanisms, and potential therapies. Mech Ageing Dev. 2003;124:287–99. - PubMed
-
- Gerstenfeld L, Einhorn T. Developmental aspects of fracture healing and the use of pharmacological agents to alter healing. J Musculoskeletal Neuronal Interact. 2003;3:297–303. - PubMed
-
- Stein A, Perren S, Cordey J, Kenwright J, Mosheiff R, Francis M. The muscle bed—a crucial factor in fracture healing: a physiological concept. Orthopedics. 2002;25:1379–1383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
