An Slfn2 mutation causes lymphoid and myeloid immunodeficiency due to loss of immune cell quiescence
- PMID: 20190759
- PMCID: PMC2861894
- DOI: 10.1038/ni.1847
An Slfn2 mutation causes lymphoid and myeloid immunodeficiency due to loss of immune cell quiescence
Abstract
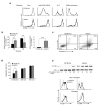
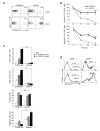
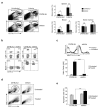
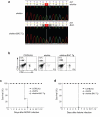
Here we describe a previously unknown form of inherited immunodeficiency revealed by an N-ethyl-N-nitrosourea-induced mutation called elektra. Mice homozygous for this mutation showed enhanced susceptibility to bacterial and viral infection and diminished numbers of T cells and inflammatory monocytes that failed to proliferate after infection and died via the intrinsic apoptotic pathway in response to diverse proliferative stimuli. They also had a greater proportion of T cells poised to replicate DNA, and their T cells expressed a subset of activation markers, suggestive of a semi-activated state. We positionally ascribe the elektra phenotype to a mutation in the gene encoding Schlafen-2 (Slfn2). Our findings identify a physiological role for Slfn2 in the defense against pathogens through the regulation of quiescence in T cells and monocytes.
Figures




Comment in
-
Quieting T cells with Slfn2.Nat Immunol. 2010 Apr;11(4):281-2. doi: 10.1038/ni0410-281. Nat Immunol. 2010. PMID: 20300134 Free PMC article. Review.
References
-
- Yusuf I, Fruman DA. Regulation of quiescence in lymphocytes. Trends Immunol. 2003;24:380–386. - PubMed
-
- Glynne R, Ghandour G, Rayner J, Mack DH, Goodnow CC. B-lymphocyte quiescence, tolerance and activation as viewed by global gene expression profiling on microarrays. Immunol. Rev. 2000;176:216–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

