Membrane transporters in drug development
- PMID: 20190787
- PMCID: PMC3326076
- DOI: 10.1038/nrd3028
Membrane transporters in drug development
Abstract
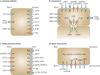
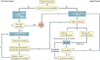
Membrane transporters can be major determinants of the pharmacokinetic, safety and efficacy profiles of drugs. This presents several key questions for drug development, including which transporters are clinically important in drug absorption and disposition, and which in vitro methods are suitable for studying drug interactions with these transporters. In addition, what criteria should trigger follow-up clinical studies, and which clinical studies should be conducted if needed. In this article, we provide the recommendations of the International Transporter Consortium on these issues, and present decision trees that are intended to help guide clinical studies on the currently recognized most important drug transporter interactions. The recommendations are generally intended to support clinical development and filing of a new drug application. Overall, it is advised that the timing of transporter investigations should be driven by efficacy, safety and clinical trial enrolment questions (for example, exclusion and inclusion criteria), as well as a need for further understanding of the absorption, distribution, metabolism and excretion properties of the drug molecule, and information required for drug labelling.
Figures
Comment in
-
Transporters in drug development: advancing on the Critical Path.Nat Rev Drug Discov. 2010 Mar;9(3):175-6. doi: 10.1038/nrd3124. Nat Rev Drug Discov. 2010. PMID: 20222180 No abstract available.
-
Understanding transport through pharmacological barriers--are we there yet?Nat Rev Drug Discov. 2010 Nov;9(11):897-8. doi: 10.1038/nrd3187-c1. Epub 2010 Oct 29. Nat Rev Drug Discov. 2010. PMID: 21031004 No abstract available.
-
The relevance of assessment of intestinal P-gp inhibition using digoxin as an in vivo probe substrate.Nat Rev Drug Discov. 2011 Jan;10(1):75; author reply 75. doi: 10.1038/nrd3028-c1. Nat Rev Drug Discov. 2011. PMID: 21193869 No abstract available.
References
-
-
Giacomini KM, Sugiyama Y. In: Gilman’s The Pharmacological Basis of Therapeutics. Brunton LL, Lazo JS, Parker RL, editors. New York: McGraw-Hill; 2006. pp. 41–70. This chapter in a textbook provides an excellent overview of transporters.
-
-
-
Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv. Drug Deliv. Rev. 2003;55:3–29. This manuscript provides an excellent review of ABC transporters that are important in drug response.
-
-
- Sai Y. Biochemical and molecular pharmacological aspects of transporters as determinants of drug disposition. Drug Metab. Pharmacokinet. 2005;20:91–99. - PubMed
-
- Cascorbi I. Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol. Ther. 2006;112:457–473. - PubMed
-
- Choudhuri S, Klaassen CD. Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int. J. Toxicol. 2006;25:231–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

