Neurotoxic manifestations of an overdose intrathecal injection of gadopentetate dimeglumine
- PMID: 20191058
- PMCID: PMC2826734
- DOI: 10.3346/jkms.2010.25.3.505
Neurotoxic manifestations of an overdose intrathecal injection of gadopentetate dimeglumine
Erratum in
- J Korean Med Sci. 2010 Apr;25(4):656
Abstract
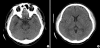
The intravenous administration of gadopentetate dimeglumine (GD) is relatively safe and rarely causes systemic toxicity in the course of routine imaging studies. However, the general safety of intrathecal GD has not been established. We report a very rare case of an overdose intrathecal GD injection presenting with neurotoxic manifestations, including a decreased level of consciousness, global aphasia, rigidity, and visual disturbance.
Keywords: Gadolinium DTPA; Injections, Spinal; Neurologic Manifestations.
Figures


Similar articles
-
Gadolinium encephalopathy due to accidental intrathecal administration of gadopentetate dimeglumine.J Neurol. 2007 Jun;254(6):810-2. doi: 10.1007/s00415-006-0439-x. Epub 2007 Apr 2. J Neurol. 2007. PMID: 17401744 No abstract available.
-
Neurotoxic effects of gadopentetate dimeglumine: behavioral disturbance and morphology after intracerebroventricular injection in rats.AJNR Am J Neuroradiol. 1996 Feb;17(2):365-73. AJNR Am J Neuroradiol. 1996. PMID: 8938312 Free PMC article.
-
Neurotoxic potential of gadodiamide after injection into the lateral cerebral ventricle of rats.AJNR Am J Neuroradiol. 1998 Sep;19(8):1455-62. AJNR Am J Neuroradiol. 1998. PMID: 9763378 Free PMC article.
-
Intrathecal gadolinium (gadopentetate dimeglumine)-enhanced MR cisternography used to determine potential communication between the cerebrospinal fluid pathways and intracranial arachnoid cysts.Neuroradiology. 2004 Sep;46(9):744-54. doi: 10.1007/s00234-004-1240-0. Neuroradiology. 2004. PMID: 15289956 Clinical Trial.
-
Critical Questions Regarding Gadolinium Deposition in the Brain and Body After Injections of the Gadolinium-Based Contrast Agents, Safety, and Clinical Recommendations in Consideration of the EMA's Pharmacovigilance and Risk Assessment Committee Recommendation for Suspension of the Marketing Authorizations for 4 Linear Agents.Invest Radiol. 2017 Jun;52(6):317-323. doi: 10.1097/RLI.0000000000000374. Invest Radiol. 2017. PMID: 28368880 Review.
Cited by
-
Children With Intracranial Arachnoid Cysts: Classification and Treatment.Medicine (Baltimore). 2015 Nov;94(44):e1749. doi: 10.1097/MD.0000000000001749. Medicine (Baltimore). 2015. PMID: 26554773 Free PMC article. Clinical Trial.
-
Are gadolinium-based contrast media safe alternatives to iodinated contrast agents for the safe performance of spinal injection procedures?Interv Pain Med. 2025 Jan 2;4(1):100538. doi: 10.1016/j.inpm.2024.100538. eCollection 2025 Mar. Interv Pain Med. 2025. PMID: 40226043 Free PMC article.
-
The safety of magnetic resonance imaging contrast agents.Front Toxicol. 2024 Aug 12;6:1376587. doi: 10.3389/ftox.2024.1376587. eCollection 2024. Front Toxicol. 2024. PMID: 39188505 Free PMC article.
-
Neurotoxic manifestations of high-dose intrathecal gadolinium administration for CT myelogram.Radiol Case Rep. 2020 Aug 22;15(10):1992-1995. doi: 10.1016/j.radcr.2020.07.084. eCollection 2020 Oct. Radiol Case Rep. 2020. PMID: 32874398 Free PMC article.
-
MR myelography for identification of spinal CSF leak in spontaneous intracranial hypotension.AJNR Am J Neuroradiol. 2014 Oct;35(10):2007-12. doi: 10.3174/ajnr.A3975. Epub 2014 May 22. AJNR Am J Neuroradiol. 2014. PMID: 24852289 Free PMC article.
References
-
- Maramattom BV, Manno EM, Wijdicks EF, Lindell EP. Gadolinium encephalopathy in a patient with renal failure. Neurology. 2005;64:1276–1278. - PubMed
-
- Tali ET, Ercan N, Krumina G, Rudwan M, Mironov A, Zeng QY, Jinkins JR. Intrathecal gadolinium (gadopentetate dimeglumine) enhanced magnetic resonance myelography and cisternography: results of a multicenter study. Invest Radiol. 2002;37:152–159. - PubMed
-
- Niendorf HP, Haustein J, Cornelius I, Alhassan A, Clauss W. Safety of gadolinium-DTPA: extended clinical experience. Magn Reson Med. 1991;22:222–228. - PubMed
-
- Goldstein HA, Kashanian FK, Blumetti RF, Holyoak WL, Hugo FP, Blumenfield DM. Safety assessment of gadopentetate dimeglumine in U.S. clinical trials. Radiology. 1990;174:17–23. - PubMed
-
- Zeng Q, Xiong L, Jinkins JR, Fan Z, Liu Z. Intrathecal gadolinium-enhanced MR myelography and cisternography: a pilot study in human patients. AJR Am J Roentgenol. 1999;173:1109–1115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

