Fabrication of Microbeads with a Controllable Hollow Interior and Porous Wall Using a Capillary Fluidic Device
- PMID: 20191106
- PMCID: PMC2828740
- DOI: 10.1002/adfm.200900763
Fabrication of Microbeads with a Controllable Hollow Interior and Porous Wall Using a Capillary Fluidic Device
Abstract
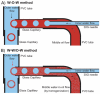
Poly(d,l-lactide-co-glycolide) (PLGA) microbeads with a hollow interior and porous wall are prepared using a simple fluidic device fabricated with PVC tubes, glass capillaries, and a needle. Using the fluidic device with three flow channels, uniform water-in-oil-in-water (W-O-W) emulsions with a single inner water droplet can be achieved with controllable dimensions by varying the flow rate of each phase. The resultant W-O-W emulsions evolve into PLGA microbeads with a hollow interior and porous wall after the organic solvent in the middle oil phase evaporates. Two approaches are employed for developing a porous structure in the wall: emulsion templating and fast solvent evaporation. For emulsion templating, a homogenized, water-in-oil (W/O) emulsion is introduced as the middle phase instead of the pure oil phase. Low-molecular-weight fluorescein isothiocyanate (FITC) and high-molecular-weight fluorescein isothiocyanate-dextran conjugate (FITC-DEX) is added to the inner water phase to elucidate both the pore size and their interconnectivity in the wall of the microbeads. From optical fluorescence microscopy and scanning electron microscopy images, it is confirmed that the emulsion-templated microbeads (W-W/O-W) have larger and better interconnected pores than the W-O-W microbeads. These microstructured microbeads can potentially be employed for cell encapsulation and tissue engineering, as well as protection of active agents.
Figures






References
-
- Lee M-H, Oh S-G, Moon S-K, Bae S-Y. J. Colloid Interface Sci. 2001;240:83. - PubMed
-
- Ferreira LAM, Seiller M, Grossiord JL, Marty JP, Wepierre J. Int. J. Pharm. 1994;109:251.
-
- Nakano M. Adv. Drug Delivery Rev. 2000;45:1. - PubMed
-
- Vasiljevic D, Parojcic J, Primorac M, Vuleta G. Int. J. Pharm. 2006;309:171. - PubMed
-
- Köster S, Angilè FE, Duan H, Agresti JJ, Wintner A, Schmitz C, Rowat AC, Merten CA, Pisignano D, Griffiths AD, Weitz DA. Lab Chip. 2008;8:1110. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
