Inhibition of Staphylococcus epidermidis biofilms using polymerizable vancomycin derivatives
- PMID: 20191335
- PMCID: PMC2895847
- DOI: 10.1007/s11999-010-1266-z
Inhibition of Staphylococcus epidermidis biofilms using polymerizable vancomycin derivatives
Abstract
Background: Biofilm formation on indwelling medical devices is a ubiquitous problem causing considerable patient morbidity and mortality. In orthopaedic surgery, this problem is exacerbated by the large number and variety of material types that are implanted. Metallic hardware in conjunction with polymethylmethacrylate (PMMA) bone cement is commonly used.
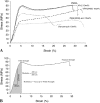
Questions/purposes: We asked whether polymerizable derivatives of vancomycin might be useful to (1) surface modify Ti-6Al-4V alloy and to surface/bulk modify PMMA bone cement to prevent Staphylococcus epidermidis biofilm formation and (2) whether the process altered the compressive modulus, yield strength, resilience, and/or fracture strength of cement copolymers.
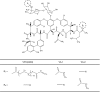
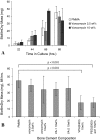
Methods: A Ti-6Al-4V alloy was silanized with methacryloxypropyltrimethoxysilane in preparation for subsequent polymer attachment. Surfaces were then coated with polymers formed from PEG(375)-acrylate or a vancomycin-PEG(3400)-PEG(375)-acrylate copolymer. PMMA was loaded with various species, including vancomycin and several polymerizable vancomycin derivatives. To assess antibiofilm properties of these materials, initial bacterial adherence to coated Ti-6Al-4V was determined by scanning electron microscopy (SEM). Biofilm dry mass was determined on PMMA coupons; the compressive mechanical properties were also determined.
Results: SEM showed the vancomycin-PEG(3400)-acrylate-type surface reduced adherent bacteria numbers by approximately fourfold when compared with PEG(375)-acrylate alone. Vancomycin-loading reduced all mechanical properties tested; in contrast, loading a vancomycin-acrylamide derivative restored these deficits but demonstrated no antibiofilm properties. A polymerizable, PEGylated vancomycin derivative reduced biofilm attachment but resulted in inferior cement mechanical properties.
Clinical relevance: The approaches presented here may offer new strategies for developing biofilm-resistant orthopaedic materials. Specifically, polymerizable derivatives of traditional antibiotics may allow for direct polymerization into existing materials such as PMMA bone cement while minimizing mechanical property compromise. Questions remain regarding ideal monomer structure(s) that confer biologic and mechanical benefits.
Figures

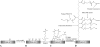


Similar articles
-
The use of quaternised chitosan-loaded PMMA to inhibit biofilm formation and downregulate the virulence-associated gene expression of antibiotic-resistant staphylococcus.Biomaterials. 2012 Jan;33(2):365-77. doi: 10.1016/j.biomaterials.2011.09.084. Epub 2011 Oct 20. Biomaterials. 2012. PMID: 22014946
-
Vancomycin derivative photopolymerized to titanium kills S. epidermidis.Clin Orthop Relat Res. 2007 Aug;461:96-105. doi: 10.1097/BLO.0b013e3180986706. Clin Orthop Relat Res. 2007. PMID: 17549033
-
Activity of bone cement loaded with daptomycin alone or in combination with gentamicin or PEG600 against Staphylococcus epidermidis biofilms.Injury. 2015 Feb;46(2):249-53. doi: 10.1016/j.injury.2014.11.014. Epub 2014 Nov 27. Injury. 2015. PMID: 25498330
-
Effect of vancomycin-coated tympanostomy tubes on methicillin-resistant Staphylococcus aureus biofilm formation: in vitro study.J Laryngol Otol. 2010 Jun;124(6):594-8. doi: 10.1017/S0022215109992672. Epub 2010 Jan 8. J Laryngol Otol. 2010. PMID: 20056010 Review.
-
Antibiofilm capacity of PMMA surfaces: A review of current knowledge.Microb Pathog. 2025 May;202:107426. doi: 10.1016/j.micpath.2025.107426. Epub 2025 Feb 25. Microb Pathog. 2025. PMID: 40015578 Review.
Cited by
-
Effects of Imipenem-containing Niosome nanoparticles against high prevalence methicillin-resistant Staphylococcus Epidermidis biofilm formed.Sci Rep. 2022 Mar 24;12(1):5140. doi: 10.1038/s41598-022-09195-9. Sci Rep. 2022. PMID: 35332241 Free PMC article.
-
Vancomycin containing PLLA/β-TCP controls experimental osteomyelitis in vivo.J Orthop Surg Res. 2014 Nov 19;9:114. doi: 10.1186/s13018-014-0114-3. J Orthop Surg Res. 2014. PMID: 25407446 Free PMC article.
-
Bacterial adhesion and growth reduction by novel rubber-derived oligomers.Biochem Biophys Res Commun. 2013 Sep 6;438(4):691-6. doi: 10.1016/j.bbrc.2013.07.120. Epub 2013 Aug 3. Biochem Biophys Res Commun. 2013. PMID: 23921230 Free PMC article.
-
Ultrasound-triggered antibiotic release from PEEK clips to prevent spinal fusion infection: Initial evaluations.Acta Biomater. 2019 Jul 15;93:12-24. doi: 10.1016/j.actbio.2019.02.041. Epub 2019 Feb 28. Acta Biomater. 2019. PMID: 30826477 Free PMC article.
-
The Impact of Incorporating Antimicrobials into Implant Surfaces.J Dent Res. 2018 Jan;97(1):14-22. doi: 10.1177/0022034517731768. Epub 2017 Sep 18. J Dent Res. 2018. PMID: 28922615 Free PMC article. Review.
References
-
- Adams K, Couch L, Cierny G, Calhoun J, Mader JT. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop Relat Res. 1992;278:244–252. - PubMed
-
- Anguita-Alonso P, Rouse MS, Piper KE, Jacofsky DJ, Osmon DR, Patel R. Comparative study of antimicrobial release kinetics from polymethylmethacrylate. Clin Orthop Relat Res. 2006;445:239–244. - PubMed
-
- Antoci V, Jr, Adams CS, Hickok NJ, Shapiro IM, Parvizi J. Vancomycin bound to Ti rods reduces periprosthetic infection: preliminary study. Clin Orthop Relat Res. 2007;461:88–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

