Effect of gaboxadol on patient-reported measures of sleep and waking function in patients with Primary Insomnia: results from two randomized, controlled, 3-month studies
- PMID: 20191935
- PMCID: PMC2823273
Effect of gaboxadol on patient-reported measures of sleep and waking function in patients with Primary Insomnia: results from two randomized, controlled, 3-month studies
Abstract
Objective: To evaluate the efficacy and safety of gaboxadol in the treatment of Primary Insomnia.
Methods: Two studies were performed in patients 18 to 65 years of age with Primary Insomnia. After a 7-day single-blind placebo run-in, patients were randomized to double-blind treatment with gaboxadol 15 mg (N = 310), 10 mg (N = 308), or placebo (N = 309) over 3 months in Study 1; and gaboxadol 15 mg (N = 304) or placebo (N = 301) over 12 months in Study 2. Treatment was administered at bedtime. The primary efficacy endpoints in each study were change from baseline in patient-reported total sleep time (sTST) and time to sleep onset (sTSO) at month 3. Safety was assessed primarily by adverse event reports.
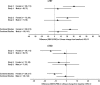
Results: In Study 1, gaboxadol 15 mg significantly improved sTST (difference vs. placebo of 20.4 min, p < 0.01) and sTSO (difference vs. placebo of -9.8 min, p < 0.05) at 3 months, while gaboxadol 10 mg had no significant effects on these measures. In Study 2, gaboxadol 15 mg showed numerical superiority for improvements on sTST (difference vs. placebo of 14.5 min) and sTSO (difference vs. placebo of -4.9 min) at 3 months, but these differences were not significant. In both studies, there was evidence that the efficacy of gaboxadol was more pronounced in women than men. Gaboxadol was generally well tolerated over 3 months in Study 1, and over 12 months in Study 2.
Conclusion: Gaboxadol 15 mg showed variable efficacy on measures of sleep duration and onset at 3 months in adult patients with Primary Insomnia in these studies and appeared to be more effective in women than men. Gaboxadol 10 mg was not effective in these studies. (Clinical trial registration numbers: NCT00103818, NCT00095069).
Figures
Similar articles
-
Effect of gaboxadol on sleep in adult and elderly patients with primary insomnia: results from two randomized, placebo-controlled, 30-night polysomnography studies.Sleep. 2008 Oct;31(10):1359-70. Sleep. 2008. PMID: 18853933 Free PMC article. Clinical Trial.
-
Effect of short-term treatment with gaboxadol on sleep maintenance and initiation in patients with primary insomnia.Sleep. 2007 Mar;30(3):281-7. doi: 10.1093/sleep/30.3.281. Sleep. 2007. PMID: 17425224 Clinical Trial.
-
A 2-week efficacy and safety study of gaboxadol and zolpidem using electronic diaries in primary insomnia outpatients.Sleep Med. 2009 Aug;10(7):705-12. doi: 10.1016/j.sleep.2008.09.010. Epub 2009 Apr 5. Sleep Med. 2009. PMID: 19346160 Clinical Trial.
-
Eszopiclone for the treatment of primary insomnia: a systematic review and meta-analysis of double-blind, randomized, placebo-controlled trials.Sleep Med. 2019 Oct;62:6-13. doi: 10.1016/j.sleep.2019.03.016. Epub 2019 Apr 6. Sleep Med. 2019. PMID: 31518944
-
Gaboxadol--a new awakening in sleep.Curr Opin Pharmacol. 2006 Feb;6(1):30-6. doi: 10.1016/j.coph.2005.10.004. Epub 2005 Dec 20. Curr Opin Pharmacol. 2006. PMID: 16368265 Review.
Cited by
-
The Comparative Effectiveness and Safety of Insomnia Drugs: A Systematic Review and Network Meta-Analysis of 153 Randomized Trials.Drugs. 2023 May;83(7):587-619. doi: 10.1007/s40265-023-01859-8. Epub 2023 Mar 22. Drugs. 2023. PMID: 36947394
-
Effects of early-life exposure to THIP on phenotype development in a mouse model of Rett syndrome.J Neurodev Disord. 2016 Oct 19;8:37. doi: 10.1186/s11689-016-9169-2. eCollection 2016. J Neurodev Disord. 2016. PMID: 27777634 Free PMC article.
-
EEG power spectra response to a 4-h phase advance and gaboxadol treatment in 822 men and women.J Clin Sleep Med. 2011 Oct 15;7(5):493-501A. doi: 10.5664/JCSM.1316. J Clin Sleep Med. 2011. PMID: 22003345 Free PMC article. Clinical Trial.
-
A study of subunit selectivity, mechanism and site of action of the delta selective compound 2 (DS2) at human recombinant and rodent native GABA(A) receptors.Br J Pharmacol. 2013 Mar;168(5):1118-32. doi: 10.1111/bph.12001. Br J Pharmacol. 2013. PMID: 23061935 Free PMC article.
-
Gaboxadol in Fragile X Syndrome: A 12-Week Randomized, Double-Blind, Parallel-Group, Phase 2a Study.Front Pharmacol. 2021 Oct 8;12:757825. doi: 10.3389/fphar.2021.757825. eCollection 2021. Front Pharmacol. 2021. PMID: 34690787 Free PMC article.
References
-
- Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93. - PubMed
-
- Wafford KA, Ebert B. Gaboxadol--a new awakening in sleep. Curr Opin Pharmacol. 2006;6:30–6. - PubMed
-
- Ebert B, Wafford KA, Deacon S. Treating insomnia: current and investigational pharmacological approaches. Pharmacol Ther. 2006;112:612–29. - PubMed
-
- Deacon S, Staner L, Staner C, Legters A, Loft H, Lundahl J. Effect of short-term treatment with gaboxadol on sleep maintenance and initiation in patients with primary insomnia. Sleep. 2007;30:281–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical