Metal-directed protein self-assembly
- PMID: 20192262
- PMCID: PMC2873059
- DOI: 10.1021/ar900273t
Metal-directed protein self-assembly
Abstract
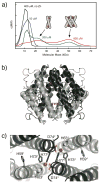
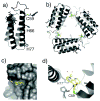
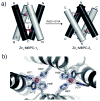
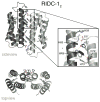
Proteins are nature's premier building blocks for constructing sophisticated nanoscale architectures that carry out complex tasks and chemical transformations. Some 70%-80% of all proteins are thought to be permanently oligomeric; that is, they are composed of multiple proteins that are held together in precise spatial organization through noncovalent interactions. Although it is of great fundamental interest to understand the physicochemical basis of protein self-assembly, the mastery of protein-protein interactions (PPIs) would also allow access to novel biomaterials with nature's favorite and most versatile building block. In this Account, we describe a new approach we have developed with this possibility in mind, metal-directed protein self-assembly (MDPSA), which utilizes the strength, directionality, and selectivity of metal-ligand interactions to control PPIs. At its core, MDPSA is inspired by supramolecular coordination chemistry, which exploits metal coordination for the self-assembly of small molecules into discrete, more-or-less predictable higher order structures. Proteins, however, are not exactly small molecules or simple metal ligands: they feature extensive, heterogeneous surfaces that can interact with each other and with metal ions in unpredictable ways. We begin by first describing the challenges of using entire proteins as molecular building blocks. We follow with an examination of our work on a model protein (cytochrome cb(562)), highlighting challenges toward establishing ground rules for MDPSA as well as progress in overcoming these challenges. Proteins are also nature's metal ligands of choice. In MDPSA, once metal ions guide proteins into forming large assemblies, they are by definition embedded within extensive interfaces formed between protein surfaces. These complex surfaces make an inorganic chemist's life somewhat difficult, yet they also provide a wide platform to modulate the metal coordination environment through distant, noncovalent interactions, exactly as natural metalloproteins and enzymes do. We describe our computational and experimental efforts toward restructuring the noncovalent interaction network formed between proteins surrounding the interfacial metal centers. This approach, of metal templating followed by the redesign of protein interfaces (metal-templated interface redesign, MeTIR), not only provides a route to engineer de novo PPIs and novel metal coordination environments but also suggests possible parallels with the evolution of metalloproteins.
Figures
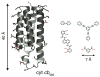
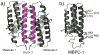





References
-
- Kortemme T, Baker D. Computational design of protein-protein interactions. Curr Opin Chem Biol. 2004;8:91–97. - PubMed
-
- Leininger S, Olenyuk B, Stang PJ. Self-assembly of discrete cyclic nanostructures mediated by transition metals. Chem Rev. 2000;100:853–907. - PubMed
-
- Holliday BJ, Mirkin CA. Strategies for the construction of supramolecular compounds through coordination chemistry. Angew Chem, Int Ed Eng. 2001;40:2022–2043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

