Lessons learned from UvrD helicase: mechanism for directional movement
- PMID: 20192763
- PMCID: PMC3480338
- DOI: 10.1146/annurev.biophys.093008.131415
Lessons learned from UvrD helicase: mechanism for directional movement
Abstract
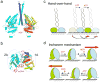
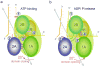
How do molecular motors convert chemical energy to mechanical work? Helicases and nucleic acids offer simple motor systems for extensive biochemical and biophysical analyses. Atomic resolution structures of UvrD-like helicases complexed with DNA in the presence of AMPPNP, ADP.Pi, and Pi reveal several salient points that aid our understanding of mechanochemical coupling. Each ATPase cycle causes two motor domains to rotationally close and open. At a minimum, two motor-track contact points of alternating tight and loose attachment convert domain rotations to unidirectional movement. A motor is poised for action only when fully in contact with its track and, if applicable, working against a load. The orientation of domain rotation relative to the track determines whether the movement is linear, spiral, or circular. Motors powered by ATPases likely deliver each power stroke in two parts, before and after ATP hydrolysis. Implications of these findings for analyzing hexameric helicase, F(1)F(0) ATPase, and kinesin are discussed.
Figures
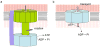
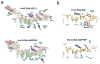
References
-
- Abrahams JP, Leslie AG, Lutter R, Walker JE. Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–8. - PubMed
-
- Ali JA, Lohman TM. Kinetic measurement of the step size of DNA unwinding by Escherichia coli UvrD helicase. Science. 1997;275:377–80. - PubMed
-
- Amos LA, van den Ent F, Lowe J. Structural/functional homology between the bacterial and eukaryotic cytoskeletons. Curr Opin Cell Biol. 2004;16:24–31. - PubMed
-
- Andersen CB, Ballut L, Johansen JS, Chamieh H, Nielsen KH, et al. Structure of the exon junction core complex with a trapped DEAD-box ATPase bound to RNA. Science. 2006;313:1968–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

