Optical control of neuronal activity
- PMID: 20192766
- PMCID: PMC7584471
- DOI: 10.1146/annurev.biophys.093008.131400
Optical control of neuronal activity
Abstract
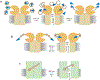
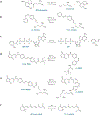
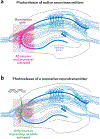
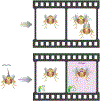
Advances in optics, genetics, and chemistry have enabled the investigation of brain function at all levels, from intracellular signals to single synapses, whole cells, circuits, and behavior. Until recent years, these research tools have been utilized in an observational capacity: imaging neural activity with fluorescent reporters, for example, or correlating aberrant neural activity with loss-of-function and gain-of-function pharmacological or genetic manipulations. However, optics, genetics, and chemistry have now combined to yield a new strategy: using light to drive and halt neuronal activity with molecular specificity and millisecond precision. Photostimulation of neurons is noninvasive, has unmatched spatial and temporal resolution, and can be targeted to specific classes of neurons. The optical methods developed to date encompass a broad array of strategies, including photorelease of caged neurotransmitters, engineered light-gated receptors and channels, and naturally light-sensitive ion channels and pumps. In this review, we describe photostimulation methods, their applications, and opportunities for further advancement.
Figures

References
-
- Ahmed NA, Radwan NM, Ibrahim KM, Khedr ME, El Aziz MA, Khadrawy YA. 2008. Effect of three different intensities of infrared laser energy on the levels of amino acid neurotransmitters in the cortex and hippocampus of rat brain. Photomed. Laser Surg 26(5):479–88 - PubMed
-
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. 2009. Temporally precise in vivo control of intracellular signalling. Nature 458(7241):1025–29 - PubMed
-
- Allégre G, Avrillier S, Albe-Fessard D. 1994. Stimulation in the rat of a nerve fiber bundle by a short UV pulse from an excimer laser. Neurosci. Lett 180(2):261–64 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

