Rewiring cells: synthetic biology as a tool to interrogate the organizational principles of living systems
- PMID: 20192780
- PMCID: PMC2965450
- DOI: 10.1146/annurev.biophys.050708.133652
Rewiring cells: synthetic biology as a tool to interrogate the organizational principles of living systems
Abstract
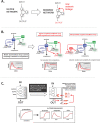
The living cell is an incredibly complex entity, and the goal of predictively and quantitatively understanding its function is one of the next great challenges in biology. Much of what we know about the cell concerns its constituent parts, but to a great extent we have yet to decode how these parts are organized to yield complex physiological function. Classically, we have learned about the organization of cellular networks by disrupting them through genetic or chemical means. The emerging discipline of synthetic biology offers an additional, powerful approach to study systems. By rearranging the parts that comprise existing networks, we can gain valuable insight into the hierarchical logic of the networks and identify the modular building blocks that evolution uses to generate innovative function. In addition, by building minimal toy networks, one can systematically explore the relationship between network structure and function. Here, we outline recent work that uses synthetic biology approaches to investigate the organization and function of cellular networks, and describe a vision for a synthetic biology toolkit that could be used to interrogate the design principles of diverse systems.
Figures





References
-
- Alon U. Network motifs: theory and experimental approaches. Nature Reviews Genetics. 2007;8:450. - PubMed
-
- Alves R, Savageau M. Extending the method of mathematically controlled comparison to include numerical comprisons. Bioinformatics. 2000;16:786. - PubMed
-
- Atkinson M, Savageau M, Myers J, Ninfa A. Development of genetic circuitry exhibiting toggle switch or socillatory behavior in Escherichia coli. Cell. 2003;113:597. - PubMed
-
- Barkai N, Leibler S. Biological rhythms: Circadian clocks limited by noise. Nature. 2000;403:267. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

