Attention, intention, and priority in the parietal lobe
- PMID: 20192813
- PMCID: PMC3683564
- DOI: 10.1146/annurev-neuro-060909-152823
Attention, intention, and priority in the parietal lobe
Abstract
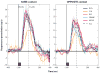
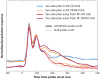
For many years there has been a debate about the role of the parietal lobe in the generation of behavior. Does it generate movement plans (intention) or choose objects in the environment for further processing? To answer this, we focus on the lateral intraparietal area (LIP), an area that has been shown to play independent roles in target selection for saccades and the generation of visual attention. Based on results from a variety of tasks, we propose that LIP acts as a priority map in which objects are represented by activity proportional to their behavioral priority. We present evidence to show that the priority map combines bottom-up inputs like a rapid visual response with an array of top-down signals like a saccade plan. The spatial location representing the peak of the map is used by the oculomotor system to target saccades and by the visual system to guide visual attention.
Conflict of interest statement
The authors are not aware of any affiliations, memberships, funding, financial holdings, or any other conflicts of interests that might be perceived as affecting the objectivity of this review.
Figures





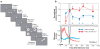

References
-
- Andersen RA, Asanuma C, Cowan WM. Callosal and prefrontal associational projecting cell populations in area 7a of the macaque monkey: a study using retrogradely transported fluorescent dyes. J Comp Neurol. 1985;232:443–55. - PubMed
-
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. - PubMed
-
- Baizer JS, Desimone R, Ungerleider LG. Comparison of subcortical connections of inferior temporal and posterior parietal cortex in monkeys. Visual Neurosci. 1993;10:59–72. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

