Fluctuations in biological and bioinspired electron-transfer reactions
- PMID: 20192814
- PMCID: PMC2883780
- DOI: 10.1146/annurev.physchem.012809.103436
Fluctuations in biological and bioinspired electron-transfer reactions
Abstract
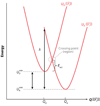
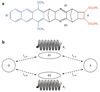
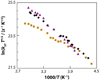
Central to theories of electron transfer (ET) is the idea that nuclear motion generates a transition state that enables electron flow to proceed, but nuclear motion also induces fluctuations in the donor-acceptor (DA) electronic coupling that is the rate-limiting parameter for nonadiabatic ET. The interplay between the DA energy gap and DA coupling fluctuations is particularly noteworthy in biological ET, where flexible protein and mobile water bridges take center stage. Here, we discuss the critical timescales at play for ET reactions in fluctuating media, highlighting issues of the Condon approximation, average medium versus fluctuation-controlled electron tunneling, gated and solvent relaxation controlled electron transfer, and the influence of inelastic tunneling on electronic coupling pathway interferences. Taken together, one may use this framework to establish principles to describe how macromolecular structure and structural fluctuations influence ET reactions. This framework deepens our understanding of ET chemistry in fluctuating media. Moreover, it provides a unifying perspective for biophysical charge-transfer processes and helps to frame new questions associated with energy harvesting and transduction in fluctuating media.
Figures





Similar articles
-
Steering electrons on moving pathways.Acc Chem Res. 2009 Oct 20;42(10):1669-78. doi: 10.1021/ar900123t. Acc Chem Res. 2009. PMID: 19645446 Free PMC article. Review.
-
Coherence in electron transfer pathways.Procedia Chem. 2011 Jan 1;3(1):99-104. doi: 10.1016/j.proche.2011.08.016. Procedia Chem. 2011. PMID: 23833692 Free PMC article.
-
Charge transfer in dynamical biosystems, or the treachery of (static) images.Acc Chem Res. 2015 Feb 17;48(2):474-81. doi: 10.1021/ar500271d. Epub 2014 Oct 13. Acc Chem Res. 2015. PMID: 25307316 Free PMC article. Review.
-
Electron transfer, decoherence, and protein dynamics: insights from atomistic simulations.Acc Chem Res. 2015 Apr 21;48(4):1090-7. doi: 10.1021/ar5002796. Epub 2015 Mar 2. Acc Chem Res. 2015. PMID: 25730126
-
Interference, fluctuation, and alternation of electron tunneling in protein media. 2. Non-condon theory for the energy gap dependence of electron transfer rate.J Phys Chem B. 2005 Aug 18;109(32):15621-35. doi: 10.1021/jp051606i. J Phys Chem B. 2005. PMID: 16852980
Cited by
-
Electron hopping through proteins.Coord Chem Rev. 2012 Nov 1;256(21-22):2478-2487. doi: 10.1016/j.ccr.2012.03.032. Epub 2012 Apr 5. Coord Chem Rev. 2012. PMID: 23420049 Free PMC article.
-
Temperature Dependence of Charge and Spin Transfer in Azurin.J Phys Chem C Nanomater Interfaces. 2021 May 13;125(18):9875-9883. doi: 10.1021/acs.jpcc.1c01218. Epub 2021 Apr 29. J Phys Chem C Nanomater Interfaces. 2021. PMID: 34055128 Free PMC article.
-
Distance-independent charge recombination kinetics in cytochrome c-cytochrome c peroxidase complexes: compensating changes in the electronic coupling and reorganization energies.J Phys Chem B. 2013 Aug 8;117(31):9129-41. doi: 10.1021/jp401551t. Epub 2013 Jul 29. J Phys Chem B. 2013. PMID: 23895339 Free PMC article.
-
Ultrafast dynamics of nonequilibrium electron transfer in photoinduced redox cycle: solvent mediation and conformation flexibility.J Phys Chem B. 2012 Aug 2;116(30):9130-40. doi: 10.1021/jp304518f. Epub 2012 Jul 19. J Phys Chem B. 2012. PMID: 22735101 Free PMC article.
-
Clustering of Aromatic Amino Acid Residues around Methionine in Proteins.Biomolecules. 2021 Dec 21;12(1):6. doi: 10.3390/biom12010006. Biomolecules. 2021. PMID: 35053154 Free PMC article.
References
LITERATURE CITED
-
- Marcus RA, Sutin N. Electron transfers in chemistry and biology. Biochim. Biophys. Acta. 1985;811:265–322.
-
- Jortner J, Ratner MA. Molecular Electronics. Oxford: Blackwell Science; 1997.
-
- Kuznetsov AM, Ulstrup J. Electron Transfer in Chemistry and Biology. Chichester, UK: Wiley; 1999.
-
- Jortner J, Bixon M, editors. Electron Transfer: From Isolated Molecules to Biomolecules. Adv. Chem. Phys. Ser. New York: Wiley Intersci.; 1999. pp. 106–107.
-
- May V, Kuhn O. Charge and Energy Transfer Dynamics in Molecular Systems. Berlin: Wiley-VCH; 2000.
RELATED RESOURCES
-
- Bendall DS, editor. Protein Electron Transfer. Oxford, UK: BIOS Sci.; 1996.
-
- Bertini I, Gray HB, Stiefel EI, Valentine JS. Bioinorganical Inorganic Chemistry: Structure and Reactivity. Sausalito, CA: Univ. Sci.; 2007.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

