Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency
- PMID: 20192887
- PMCID: PMC3085903
- DOI: 10.1515/jpm.2010.040
Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency
Abstract
Aims: The purpose of this study was to determine the clinical significance of detecting microbial footprints of ureaplasmas in amniotic fluid (AF) using specific primers for the polymerase chain reaction (PCR) in patients presenting with cervical insufficiency.
Methods: Amniocentesis was performed in 58 patients with acute cervical insufficiency (cervical dilatation, > or =1.5 cm) and intact membranes, and without regular contractions (gestational age, 16-29 weeks). AF was cultured for aerobic and anaerobic bacteria as well as genital mycoplasmas. Ureaplasmas (Ureaplasma urealyticum and Ureaplasma parvum) were detected by PCR using specific primers. Patients were divided into three groups according to the results of AF culture and PCR for ureaplasmas: those with a negative AF culture and a negative PCR (n=44), those with a negative AF culture and a positive PCR (n=10), and those with a positive AF culture regardless of PCR result (n=4).
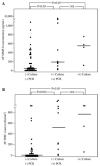
Results: 1) Ureaplasmas were detected by PCR in 19.0% (11/58) of patients, by culture in 5.2% (3/58), and by culture and/or PCR in 22.4% (13/58); 2) Among the 11 patients with a positive PCR for ureaplasmas, the AF culture was negative in 91% (10/11); 3) Patients with a negative AF culture and a positive PCR for ureaplasmas had a significantly higher median AF matrix metalloproteinase-8 (MMP-8) concentration and white blood cell (WBC) count than those with a negative AF culture and a negative PCR (P<0.001 and P<0.05, respectively); 4) Patients with a positive PCR for ureaplasmas but a negative AF culture had a higher rate of spontaneous preterm birth within two weeks of amniocentesis than those with a negative AF culture and a negative PCR (P<0.05 after adjusting for gestational age at amnio-centesis); 5) Of the patients who delivered within two weeks of amniocentesis, those with a positive PCR for ureaplasmas and a negative AF culture had higher rates of histologic amnionitis and funisitis than those with a negative AF culture and a negative PCR (P<0.05 after adjusting for gestational age at amniocentesis, for each); 6) However, no significant differences in the intensity of the intra-amniotic inflammatory response and perinatal outcome were found between patients with a positive AF culture and those with a negative AF culture and a positive PCR.
Conclusions: 1) Cultivation techniques for ureaplasmas did not detect most cases of intra-amniotic infection caused by these microorganisms (91% of cases with cervical insufficiency and microbial footprints for ureaplasmas in the amniotic cavity had a negative AF culture); 2) Patients with a negative AF culture and a positive PCR assay were at risk for intra-amniotic and fetal inflammation as well as spontaneous preterm birth.
Conflict of interest statement
The authors stated that there are no conflicts of interest regarding the publication of this article.
Figures
Similar articles
-
Evidence that antibiotic administration is effective in the treatment of a subset of patients with intra-amniotic infection/inflammation presenting with cervical insufficiency.Am J Obstet Gynecol. 2019 Aug;221(2):140.e1-140.e18. doi: 10.1016/j.ajog.2019.03.017. Epub 2019 Mar 28. Am J Obstet Gynecol. 2019. PMID: 30928565 Free PMC article.
-
The involvement of human amnion in histologic chorioamnionitis is an indicator that a fetal and an intra-amniotic inflammatory response is more likely and severe: clinical implications.Placenta. 2009 Jan;30(1):56-61. doi: 10.1016/j.placenta.2008.09.017. Epub 2008 Nov 28. Placenta. 2009. PMID: 19046766 Free PMC article.
-
Gastric fluid versus amniotic fluid analysis for the identification of intra-amniotic infection due to Ureaplasma species.J Matern Fetal Neonatal Med. 2016;29(16):2579-87. doi: 10.3109/14767058.2015.1098614. Epub 2015 Dec 2. J Matern Fetal Neonatal Med. 2016. PMID: 26631980 Free PMC article.
-
Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation.Am J Obstet Gynecol. 2017 Jun;216(6):604.e1-604.e11. doi: 10.1016/j.ajog.2017.02.035. Epub 2017 Feb 28. Am J Obstet Gynecol. 2017. PMID: 28257964 Free PMC article.
-
Amniotic fluid sludge as a marker of intra-amniotic infection and histological chorioamnionitis in cervical insufficiency: a report of four cases and literature review.J Matern Fetal Neonatal Med. 2016;29(16):2681-4. doi: 10.3109/14767058.2015.1101445. Epub 2015 Nov 9. J Matern Fetal Neonatal Med. 2016. PMID: 26553434 Review.
Cited by
-
Genital Mycoplasmas and Biomarkers of Inflammation and Their Association With Spontaneous Preterm Birth and Preterm Prelabor Rupture of Membranes: A Systematic Review and Meta-Analysis.Front Microbiol. 2022 Mar 30;13:859732. doi: 10.3389/fmicb.2022.859732. eCollection 2022. Front Microbiol. 2022. PMID: 35432251 Free PMC article. Review.
-
The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women.Microbiome. 2014 Feb 3;2(1):4. doi: 10.1186/2049-2618-2-4. Microbiome. 2014. PMID: 24484853 Free PMC article.
-
A metagenomic study of biliary microbiome change along the cholecystitis-carcinoma sequence.Clin Transl Med. 2020 Jun;10(2):e97. doi: 10.1002/ctm2.97. Epub 2020 Jun 11. Clin Transl Med. 2020. PMID: 32526082 Free PMC article.
-
The clinical significance of a positive Amnisure test in women with preterm labor and intact membranes.J Matern Fetal Neonatal Med. 2012 Sep;25(9):1690-8. doi: 10.3109/14767058.2012.657279. Epub 2012 Apr 25. J Matern Fetal Neonatal Med. 2012. PMID: 22280400 Free PMC article.
-
The diagnostic performance of the Mass Restricted (MR) score in the identification of microbial invasion of the amniotic cavity or intra-amniotic inflammation is not superior to amniotic fluid interleukin-6.J Matern Fetal Neonatal Med. 2014 May;27(8):757-69. doi: 10.3109/14767058.2013.844123. Epub 2013 Dec 16. J Matern Fetal Neonatal Med. 2014. PMID: 24028673 Free PMC article.
References
-
- Aaltonen R, Heikkinen J, Vahlberg T, Jensen JS, Alanen A. Local inflammatory response in choriodecidua induced by ureaplasma urealyticum. Br J Obstet Gynecol. 2007;114:1432–5. - PubMed
-
- Abele-Horn M, Wolff C, Dressel P, Zimmermann A, Vahlensieck W, Pfaff F, et al. Polymerase chain reaction versus culture for detection of ureaplasma urealyticum and mycoplasma hominis in the urogenital tract of adults and the respiratory tract of newborns. Eur J Clin Microbiol Infect Dis. 1996;15:595–8. - PubMed
-
- Bashiri A, Horowitz S, Huleihel M, Hackmon R, Dukler D, Mazor M. Elevated concentrations of interleukin-6 in intra-amniotic infection with ureaplasma urealyticum in asymptomatic women during genetic amniocentesis. Acta Obstet Gynecol Scand. 1999;78:379–82. - PubMed
-
- Blanchard A, Hentschel J, Duffy L, Baldus K, Cassell GH. Detection of ureaplasma urealyticum by polymerase chain reaction in the urogenital tract of adults, in amniotic fluid, and in the respiratory tract of newborns. Clin Infect Dis. 1993;17(Suppl 1):S148–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
