From HLA-B27 to spondyloarthritis: a journey through the ER
- PMID: 20193000
- PMCID: PMC2912611
- DOI: 10.1111/j.0105-2896.2009.00865.x
From HLA-B27 to spondyloarthritis: a journey through the ER
Abstract
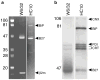
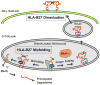
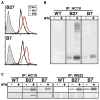
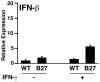
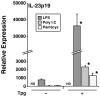
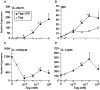
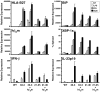
Almost four decades of research into the role of human leukocyte antigen-B27 (HLA-B27) in susceptibility to spondyloarthritis has yet to yield a convincing answer. New results from an HLA-B27 transgenic rat model now demonstrate quite convincingly that CD8(+) T cells are not required for the inflammatory phenotype. Discoveries that the HLA-B27 heavy chain has a tendency to misfold during the assembly of class I complexes in the endoplasmic reticulum (ER) and to form aberrant disulfide-linked dimers after transport to the cell surface have forced the generation of new ideas about its role in disease pathogenesis. In transgenic rats, HLA-B27 misfolding generates ER stress and leads to activation of the unfolded protein response, which dramatically enhances the production of interleukin-23 (IL-23) in response to pattern recognition receptor agonists. These findings have led to the discovery of striking T-helper 17 cell activation and expansion in this animal model, consistent with results emerging from humans with spondyloarthritis and the discovery of IL23R as an additional susceptibility gene for ankylosing spondylitis. Together, these results suggest a novel link between HLA-B27 and the T-helper 17 axis through the consequences of protein misfolding and open new avenues of investigation as well as identifying new targets for therapeutic intervention in this group of diseases.
Figures

References
-
- Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. - PubMed
-
- Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379–1390. - PubMed
-
- Caffrey MF, James DC. Human lymphocyte antigen association in ankylosing spondylitis. Nature. 1973;242:121. - PubMed
-
- Brewerton DA, Hart FD, Nicholls A, Caffrey M, James DCO, Sturrock RD. Ankylosing spondylitis and HL-A 27. Lancet. 1973;1:904–907. - PubMed
-
- Schlosstein L, Terasaki PI, Bluestone R, Pearson CM. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med. 1973;288:704–706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

