Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance
- PMID: 20193017
- PMCID: PMC3507434
- DOI: 10.1111/j.0105-2896.2009.00881.x
Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance
Abstract
Plasmacytoid dendritic cells (pDCs) are bone marrow-derived cells that secrete large amounts of type I interferon (IFN) in response to viruses. Type I IFNs are pleiotropic cytokines with antiviral activity that also enhance innate and adaptive immune responses. Viruses trigger activation of pDCs and type I IFN responses mainly through the Toll-like receptor pathway. However, a variety of activating and inhibitory pDC receptors fine tune the amplitude of type I IFN responses. Chronic activation and secretion of type I IFN in the absence of infection can promote autoimmune diseases. Furthermore, while activated pDCs promote immunity and autoimmunity, resting or alternatively activated pDCs may be tolerogenic. The various roles of pDCs have been extensively studied in vitro and in vivo with depleting antibodies. However, depleting antibodies cross-react with other cell types that are critical for eliciting protective immunity, potentially yielding ambiguous phenotypes. Here we discuss new approaches to assess pDC functions in vivo and provide preliminary data on their potential roles during viral infections. Such approaches would also prove useful in the more specific evaluation of how pDCs mediate tolerance and autoimmunity. Finally, we discuss the emergent role of pDCs and one of their receptors, tetherin, in human immunodeficiency virus pathogenesis.
Figures
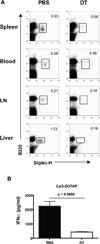


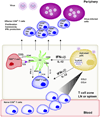
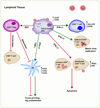

References
-
- Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. - PubMed
-
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. - PubMed
-
- Muller-Hermelink HK, Stein H, Steinmann G, Lennert K. Malignant lymphoma of plasmacytoid T-cells. Morphologic and immunologic studies characterizing a special type of T-cell. Am J Surg Pathol. 1983;7:849–862. - PubMed
-
- Lennert K, Remmele W. [Karyometric research on lymph node cells in man. I. Germinoblasts, lymphoblasts & lymphocytes.] Acta Haematol. 1958;19:99–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

