Hepatotoxicity and effectiveness of a Nevirapine-based antiretroviral therapy in HIV-infected patients with or without viral hepatitis B or C infection in Cameroon
- PMID: 20193053
- PMCID: PMC2841671
- DOI: 10.1186/1471-2458-10-105
Hepatotoxicity and effectiveness of a Nevirapine-based antiretroviral therapy in HIV-infected patients with or without viral hepatitis B or C infection in Cameroon
Abstract
Background: Coinfection with hepatitis B virus (HBV) or hepatitis C virus (HCV) in HIV-infected patients receiving a commonly used nevirapine-based antiretroviral therapy is a major concern for African clinicians owing to its high prevalence, the infrequent testing and treatment of viral hepatitis, and the impact of liver disease on the tolerability and effectiveness of anti-HIV treatment. We compared the hepatotoxicity and the immunological, virological and clinical effectiveness of a nevirapine-based antiretroviral therapy between patients infected with HIV only and patients coinfected with hepatitis B or C virus in Cameroon.
Methods: A retrospective cohort study was conducted among HIV-1-infected patients. Plasma HBV DNA and HCV RNA were tested in positive or indeterminate samples for HBsAg or HCV antibodies, respectively. All patients received nevirapine and lamivudine plus stavudine or zidovudine.
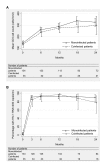
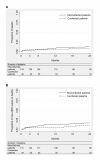
Results: Of 169 HIV-1-infected patients with a median baseline CD4 count of 135 cells/mm3 (interquartile range [IQR] 67-218), 21% were coinfected with HBV or HCV. In coinfected patients, the median viral load was 2.47 x 107 IU/mL for HBV (IQR 3680-1.59 x 108) and 928 000 IU/mL for HCV (IQR 178 400-2.06 x 106). Multivariate analyses showed that the risk of hepatotoxicity was 2-fold higher in coinfected patients (p < 0.01). The response to antiretroviral therapy was however comparable between monoinfected and coinfected patients in terms of CD4 cell count increase (p = 0.8), HIV-1 viral load below 400 copies/mL (p = 0.9), death (p = 0.3) and death or new AIDS-defining event (p = 0.1). Nevirapine was replaced by a protease inhibitor in 4 patients owing to hepatotoxicity.
Conclusion: This study suggests that the nevirapine-based antiretroviral therapy could be used safely as first-line treatment in patients with low CD4 cell count in Africa despite frequent coinfections with HBV or HCV and infrequent testing of these infections. Although testing for HBV and HCV should be systematically performed before initiating antiretroviral therapy, transaminases elevations at baseline or during treatment should be a decisive argument for testing when hepatitis status is unknown.
Figures

References
-
- Modi AA, Feld JJ. Viral hepatitis and HIV in Africa. AIDS Rev. 2007;9(1):25–39. - PubMed
-
- Stern JO, Robinson PA, Love J, Lanes S, Imperiale MS, Mayers DL. A comprehensive hepatic safety analysis of nevirapine in different populations of HIV infected patients. J Acquir Immune Defic Syndr. 2003;34(Suppl 1):S21–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

