Intermediate filaments of zebrafish retinal and optic nerve astrocytes and Müller glia: differential distribution of cytokeratin and GFAP
- PMID: 20193075
- PMCID: PMC2845598
- DOI: 10.1186/1756-0500-3-50
Intermediate filaments of zebrafish retinal and optic nerve astrocytes and Müller glia: differential distribution of cytokeratin and GFAP
Abstract
Background: Optic nerve regeneration (ONR) following injury is a model for central nervous system regeneration. In zebrafish, ONR is rapid - neurites cross the lesion and enter the optic tectum within 7 days; in mammals regeneration does not take place unless astrocytic reactivity is suppressed. Glial fibrillary acidic protein (GFAP) is used as a marker for retinal and optic nerve astrocytes in both fish and mammals, even though it has long been known that astrocytes of optic nerves in many fish, including zebrafish, express cytokeratins and not GFAP. We used immunofluorescence to localize GFAP and cytokeratin in wild-type zebrafish and transgenic zebrafish expressing green fluorescent protein (GFP) under control of a GFAP promoter to determine the pattern of expression of intermediate filaments in retina and optic nerve.
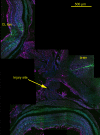
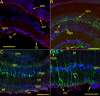
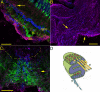
Findings: GFAP labeling and GFAP gene expression as indicated by GFP fluorescence was found only in the Müller glial cells of the retina. Within Müller cells, GFP fluorescence filled the entire cell while GFAP labelling was more restricted in distribution. No GFAP expression was observed in optic nerves. Cytokeratin labeling of astrocytes was observed throughout the optic nerve and less intensely in cells in the retinal inner plexiform layer. The retinal inner limiting membrane was strongly labeled by anti-cytokeratin.
Conclusions: Studies of astrocyte function during ONR in zebrafish cannot solely rely on GFAP as an astrocyte marker or indicator of reactivity. Future studies of ONR in zebrafish should include evaluation of changes in cytokeratin expression and localization in the optic nerve.
Figures
Similar articles
-
Activating transcription factor 3 and reactive astrocytes following optic nerve injury in zebrafish.Comp Biochem Physiol C Toxicol Pharmacol. 2012 Mar;155(2):213-8. doi: 10.1016/j.cbpc.2011.08.006. Epub 2011 Aug 24. Comp Biochem Physiol C Toxicol Pharmacol. 2012. PMID: 21889613
-
GFAP promoter drives Müller cell-specific expression in transgenic mice.Invest Ophthalmol Vis Sci. 2003 Aug;44(8):3606-13. doi: 10.1167/iovs.02-1265. Invest Ophthalmol Vis Sci. 2003. PMID: 12882814
-
Focused ultrasound as a novel strategy for noninvasive gene delivery to retinal Müller glia.Theranostics. 2020 Feb 10;10(7):2982-2999. doi: 10.7150/thno.42611. eCollection 2020. Theranostics. 2020. PMID: 32194850 Free PMC article.
-
Astrocytes as gate-keepers in optic nerve regeneration--a mini-review.Comp Biochem Physiol A Mol Integr Physiol. 2009 Feb;152(2):135-8. doi: 10.1016/j.cbpa.2008.09.026. Epub 2008 Oct 2. Comp Biochem Physiol A Mol Integr Physiol. 2009. PMID: 18930160 Review.
-
Up-regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression.Int Rev Cytol. 2003;230:263-90. doi: 10.1016/s0074-7696(03)30005-1. Int Rev Cytol. 2003. PMID: 14692684 Review.
Cited by
-
Modeling sacsin depletion in Danio Rerio offers new insight on retinal defects in ARSACS.Neurobiol Dis. 2025 Feb;205:106793. doi: 10.1016/j.nbd.2025.106793. Epub 2025 Jan 6. Neurobiol Dis. 2025. PMID: 39778749 Free PMC article.
-
The visual system of zebrafish and its use to model human ocular diseases.Dev Neurobiol. 2012 Mar;72(3):302-27. doi: 10.1002/dneu.20919. Dev Neurobiol. 2012. PMID: 21595048 Free PMC article. Review.
-
Neurodegeneration, Neuroprotection and Regeneration in the Zebrafish Retina.Cells. 2021 Mar 12;10(3):633. doi: 10.3390/cells10030633. Cells. 2021. PMID: 33809186 Free PMC article. Review.
-
Retinal Structure of Poecilia sphenops: Photoreceptor Mosaics, Synaptic Ribbon Patterns, and Glial Cell Expressions.Animals (Basel). 2024 Mar 19;14(6):939. doi: 10.3390/ani14060939. Animals (Basel). 2024. PMID: 38540038 Free PMC article.
-
Regrowth of transected retinal ganglion cell axons despite persistent astrogliosis in the lizard (Gallotia galloti).J Anat. 2013 Jul;223(1):22-37. doi: 10.1111/joa.12053. Epub 2013 May 9. J Anat. 2013. PMID: 23656528 Free PMC article.
References
-
- Bernhardt RR, Tongiorgi E, Anzini P, Schachner M. Increased expression of specific recognition molecules by retinal ganglion cells and by optic pathway glia accompanies the successful regeneration of retinal axons in adult zebrafish. J Comp Neurol. 1996;376:253–264. doi: 10.1002/(SICI)1096-9861(19961209)376:2<253::AID-CNE7>3.0.CO;2-2. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

