Dual effect of Plasmodium-infected erythrocytes on dendritic cell maturation
- PMID: 20193084
- PMCID: PMC2842284
- DOI: 10.1186/1475-2875-9-64
Dual effect of Plasmodium-infected erythrocytes on dendritic cell maturation
Abstract
Background: Infection with Plasmodium is the cause of malaria, a disease characterized by a high inflammatory response in the blood. Dendritic cells (DC) participate in both adaptive and innate immune responses, influencing the generation of inflammatory responses. DC can be activated through different receptors, which recognize specific molecules in microbes and induce the maturation of DC.
Methods: Using Plasmodium yoelii, a rodent malaria model, the effect of Plasmodium-infected erythrocytes on DC maturation and TLR responses have been analysed.
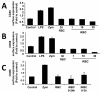
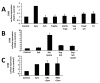
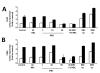
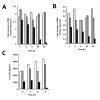
Results: It was found that intact erythrocytes infected with P. yoelii do not induce maturation of DC unless they are lysed, suggesting that accessibility of parasite inflammatory molecules to their receptors is a key issue in the activation of DC by P. yoelii. This activation is independent of MyD88. It was also observed that pre-incubation of DC with intact P. yoelii-infected erythrocytes inhibits the maturation response of DC to other TLR stimuli. The inhibition of maturation of DC is reversible, parasite-specific and increases with the stage of parasite development, with complete inhibition induced by schizonts (mature infected erythrocytes). Plasmodium yoelii-infected erythrocytes induce a broad inhibitory effect rendering DC non-responsive to ligands for TLR2, TLR3, TLR4, TLR5, TLR7 and TLR9.
Conclusions: Despite the presence of inflammatory molecules within Plasmodium-infected erythrocytes, which are probably responsible for DC maturation induced by lysates, intact Plasmodium-infected erythrocytes induce a general inhibition of TLR responsiveness in DC. The observed effect on DC could play an important role in the pathology and suboptimal immune response observed during the disease. These results help to explain why immune functions are altered during malaria, and provide a system for the identification of a parasite-derived broad inhibitor of TLR-mediated signaling pathways.
Figures




Similar articles
-
A Plasmodium yoelii soluble factor inhibits the phenotypic maturation of dendritic cells.Malar J. 2008 Dec 15;7:254. doi: 10.1186/1475-2875-7-254. Malar J. 2008. PMID: 19077314 Free PMC article.
-
Plasmodium yoelii blood-stage primes macrophage-mediated innate immune response through modulation of toll-like receptor signalling.Malar J. 2012 Apr 1;11:104. doi: 10.1186/1475-2875-11-104. Malar J. 2012. PMID: 22463100 Free PMC article.
-
Dendritic cells induce immunity and long-lasting protection against blood-stage malaria despite an in vitro parasite-induced maturation defect.Infect Immun. 2004 Sep;72(9):5331-9. doi: 10.1128/IAI.72.9.5331-5339.2004. Infect Immun. 2004. PMID: 15322030 Free PMC article.
-
Suppression of adaptive immunity to heterologous antigens during Plasmodium infection through hemozoin-induced failure of dendritic cell function.J Biol. 2006;5(2):5. doi: 10.1186/jbiol34. Epub 2006 Apr 12. J Biol. 2006. PMID: 16611373 Free PMC article. Review.
-
Immunity to erythrocytic stages of malarial parasites.Am J Trop Med Hyg. 1994;50(4 Suppl):27-32. doi: 10.4269/ajtmh.1994.50.27. Am J Trop Med Hyg. 1994. PMID: 7909653 Review.
Cited by
-
Exacerbation of autoimmune neuro-inflammation in mice cured from blood-stage Plasmodium berghei infection.PLoS One. 2014 Oct 17;9(10):e110739. doi: 10.1371/journal.pone.0110739. eCollection 2014. PLoS One. 2014. PMID: 25329161 Free PMC article.
-
Interferon regulatory factor modulation underlies the bystander suppression of malaria antigen-driven IL-12 and IFN-γ in filaria-malaria co-infection.Eur J Immunol. 2012 Mar;42(3):641-50. doi: 10.1002/eji.201141991. Epub 2012 Jan 19. Eur J Immunol. 2012. PMID: 22213332 Free PMC article.
-
A simplified, sensitive phagocytic assay for malaria cultures facilitated by flow cytometry of differentially-stained cell populations.PLoS One. 2012;7(6):e38523. doi: 10.1371/journal.pone.0038523. Epub 2012 Jun 4. PLoS One. 2012. PMID: 22675573 Free PMC article.
-
Strain-specific innate immune signaling pathways determine malaria parasitemia dynamics and host mortality.Proc Natl Acad Sci U S A. 2014 Jan 28;111(4):E511-20. doi: 10.1073/pnas.1316467111. Epub 2014 Jan 13. Proc Natl Acad Sci U S A. 2014. PMID: 24474800 Free PMC article.
-
Dendritic cells treated with crude Plasmodium berghei extracts acquire immune-modulatory properties and suppress the development of autoimmune neuroinflammation.Immunology. 2014 Oct;143(2):164-73. doi: 10.1111/imm.12298. Immunology. 2014. PMID: 24689455 Free PMC article.
References
-
- Kool M, Petrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

