Nitric oxide synthase modulates CFA-induced thermal hyperalgesia through cytokine regulation in mice
- PMID: 20193086
- PMCID: PMC2838835
- DOI: 10.1186/1744-8069-6-13
Nitric oxide synthase modulates CFA-induced thermal hyperalgesia through cytokine regulation in mice
Abstract
Background: Although it has been largely demonstrated that nitric oxide synthase (NOS), a key enzyme for nitric oxide (NO) production, modulates inflammatory pain, the molecular mechanisms underlying these effects remain to be clarified. Here we asked whether cytokines, which have well-described roles in inflammatory pain, are downstream targets of NO in inflammatory pain and which of the isoforms of NOS are involved in this process.
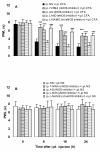
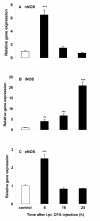
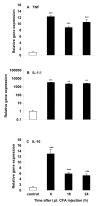
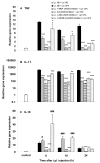
Results: Intraperitoneal (i.p.) pretreatment with 7-nitroindazole sodium salt (7-NINA, a selective neuronal NOS inhibitor), aminoguanidine hydrochloride (AG, a selective inducible NOS inhibitor), L-N(G)-nitroarginine methyl ester (L-NAME, a non-selective NOS inhibitor), but not L-N(5)-(1-iminoethyl)-ornithine (L-NIO, a selective endothelial NOS inhibitor), significantly attenuated thermal hyperalgesia induced by intraplantar (i.pl.) injection of complete Freund's adjuvant (CFA). Real-time reverse transcription-polymerase chain reaction (RT-PCR) revealed a significant increase of nNOS, iNOS, and eNOS gene expression, as well as tumor necrosis factor-alpha (TNF), interleukin-1 beta (IL-1beta), and interleukin-10 (IL-10) gene expression in plantar skin, following CFA. Pretreatment with the NOS inhibitors prevented the CFA-induced increase of the pro-inflammatory cytokines TNF and IL-1beta. The increase of the anti-inflammatory cytokine IL-10 was augmented in mice pretreated with 7-NINA or L-NAME, but reduced in mice receiving AG or L-NIO. NNOS-, iNOS- or eNOS-knockout (KO) mice had lower gene expression of TNF, IL-1beta, and IL-10 following CFA, overall corroborating the inhibitor data.
Conclusion: These findings lead us to propose that inhibition of NOS modulates inflammatory thermal hyperalgesia by regulating cytokine expression.
Figures
Similar articles
-
Differences in inflammatory pain in nNOS-, iNOS- and eNOS-deficient mice.Eur J Pain. 2007 Oct;11(7):810-8. doi: 10.1016/j.ejpain.2006.12.008. Epub 2007 Mar 28. Eur J Pain. 2007. PMID: 17395508
-
Contribution of nitric oxide synthase isoforms to cholinergic vasodilation in murine retinal arterioles.Exp Eye Res. 2013 Apr;109:60-6. doi: 10.1016/j.exer.2013.01.012. Epub 2013 Feb 19. Exp Eye Res. 2013. PMID: 23434456
-
Effect of genetic knockout or pharmacologic inhibition of neuronal nitric oxide synthase on complete Freund's adjuvant-induced persistent pain.Pain. 2005 Dec 15;119(1-3):113-123. doi: 10.1016/j.pain.2005.09.024. Epub 2005 Nov 16. Pain. 2005. PMID: 16297560
-
Nitric oxide synthases: regulation and function.Eur Heart J. 2012 Apr;33(7):829-37, 837a-837d. doi: 10.1093/eurheartj/ehr304. Epub 2011 Sep 1. Eur Heart J. 2012. PMID: 21890489 Free PMC article. Review.
-
Inducible nitric oxide synthase (iNOS): More than an inducible enzyme? Rethinking the classification of NOS isoforms.Pharmacol Res. 2025 Jun;216:107781. doi: 10.1016/j.phrs.2025.107781. Epub 2025 May 17. Pharmacol Res. 2025. PMID: 40389042 Free PMC article. Review.
Cited by
-
Small molecule inhibitors of PSD95-nNOS protein-protein interactions as novel analgesics.Neuropharmacology. 2015 Oct;97:464-75. doi: 10.1016/j.neuropharm.2015.05.038. Epub 2015 Jun 10. Neuropharmacology. 2015. PMID: 26071110 Free PMC article.
-
Sucrose-induced analgesia in mice: role of nitric oxide and opioid receptor-mediated system.Indian J Pharmacol. 2013 Nov-Dec;45(6):593-6. doi: 10.4103/0253-7613.121370. Indian J Pharmacol. 2013. PMID: 24347767 Free PMC article.
-
OCD-like behavior is caused by dysfunction of thalamo-amygdala circuits and upregulated TrkB/ERK-MAPK signaling as a result of SPRED2 deficiency.Mol Psychiatry. 2018 Feb;23(2):444-458. doi: 10.1038/mp.2016.232. Epub 2017 Jan 10. Mol Psychiatry. 2018. PMID: 28070119 Free PMC article.
-
Peripheral CaV2.2 channels in skin regulate prolonged heat hypersensitivity during neuroinflammation.bioRxiv [Preprint]. 2024 Jul 17:2024.07.13.603149. doi: 10.1101/2024.07.13.603149. bioRxiv. 2024. Update in: eNeuro. 2024 Nov 21;11(11):ENEURO.0311-24.2024. doi: 10.1523/ENEURO.0311-24.2024. PMID: 39071304 Free PMC article. Updated. Preprint.
-
Effects of pentoxifylline, 7-nitroindazole, and imipramine on tumor necrosis factor-α and indoleamine 2,3-dioxygenase enzyme activity in the hippocampus and frontal cortex of chronic mild-stress-exposed rats.Neuropsychiatr Dis Treat. 2013;9:697-708. doi: 10.2147/NDT.S41020. Epub 2013 May 24. Neuropsychiatr Dis Treat. 2013. PMID: 23785234 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

