Mutating the dileucine motif of the human beta(2)-adrenoceptor reduces the high initial rate of receptor phosphorylation by GRK without affecting postendocytic sorting
- PMID: 20193676
- PMCID: PMC3132186
- DOI: 10.1016/j.ejphar.2010.02.033
Mutating the dileucine motif of the human beta(2)-adrenoceptor reduces the high initial rate of receptor phosphorylation by GRK without affecting postendocytic sorting
Abstract
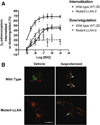
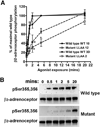
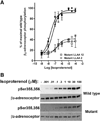
The internalization of beta(2)-adrenoceptors after agonist activation results in a desensitized and phosphorylated receptor that either resensitizes by recycling to the cell surface or becomes degraded by postendocytic sorting to lysosomes. The duration and physiological effects of agonists therefore depend on beta(2)-adrenoceptor sorting, highlighting the importance of sorting signals. Dileucine motifs within other membrane proteins act as signals for endocytosis and/or postendocytic sorting, and the beta(2)-adrenoceptor has a dileucine motif within helix 8 that might play a role in efficient receptor recycling and/or downregulation. beta(2)-adrenoceptor internalization and sorting were studied in HEK293 cells stably expressing wild type or mutant dialanine L339A,L340A beta(2)-adrenoceptors. The mutant beta(2)-adrenoceptors showed a significantly lower initial rate of phosphorylation at the prominent G-protein coupled receptor kinase (GRK) sites Ser355 and 356 compared to wild type beta(2)-adrenoceptors. Furthermore, the agonist-induced endocytic rate constant for L339A,L340A beta(2)-adrenoceptors was reduced to approximately 25% that of wild type beta(2)-adrenoceptors, which resulted in a similar reduction in agonist-induced downregulation. Internalized L339A,L340A beta(2)-adrenoceptors recycled to the surface with a rate and extent similar to that of wild type beta(2)-adrenoceptors. Therefore, although the role of L339,L340 in beta(2)-adrenoceptor recycling or postendocytic sorting seems minimal, we conclude that L339,L340 is required for the initial high rate of phosphorylation by G-protein coupled receptor kinases at Ser355,356, which in turn is required for efficient beta(2)-adrenoceptors endocytosis.
Figures



Similar articles
-
Differential phosphorylation and dephosphorylation of beta2-adrenoceptor sites Ser262 and Ser355,356.Br J Pharmacol. 2006 Feb;147(3):249-59. doi: 10.1038/sj.bjp.0706551. Br J Pharmacol. 2006. PMID: 16331289 Free PMC article.
-
Endosome sorting of beta 2-adrenoceptors is GRK5 independent.Br J Pharmacol. 2004 Jan;141(2):277-84. doi: 10.1038/sj.bjp.0705504. Epub 2003 Dec 22. Br J Pharmacol. 2004. PMID: 14691047 Free PMC article.
-
β(2)-Adrenoceptors increase translocation of GLUT4 via GPCR kinase sites in the receptor C-terminal tail.Br J Pharmacol. 2012 Mar;165(5):1442-56. doi: 10.1111/j.1476-5381.2011.01647.x. Br J Pharmacol. 2012. PMID: 21883150 Free PMC article.
-
G protein-coupled receptor-associated sorting protein 1 regulates the postendocytic sorting of seven-transmembrane-spanning G protein-coupled receptors.Pharmacology. 2010;86(1):22-9. doi: 10.1159/000314161. Epub 2010 Jun 26. Pharmacology. 2010. PMID: 20693822 Free PMC article. Review.
-
Postendocytic Sorting of Adrenergic and Opioid Receptors: New Mechanisms and Functions.Prog Mol Biol Transl Sci. 2015;132:189-206. doi: 10.1016/bs.pmbts.2015.03.005. Epub 2015 Apr 11. Prog Mol Biol Transl Sci. 2015. PMID: 26055059 Free PMC article. Review.
Cited by
-
The Role of Dileucine in the Expression and Function of Human Organic Anion Transporter 1 (hOAT1).Int J Biochem Mol Biol. 2011;2(1):31-38. Int J Biochem Mol Biol. 2011. PMID: 21494320 Free PMC article.
-
AP-3-dependent targeting of flippase ATP8A1 to lamellar bodies suppresses activation of YAP in alveolar epithelial type 2 cells.Proc Natl Acad Sci U S A. 2021 May 18;118(20):e2025208118. doi: 10.1073/pnas.2025208118. Proc Natl Acad Sci U S A. 2021. PMID: 33990468 Free PMC article.
References
-
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003;72:395–447. - PubMed
-
- Boonen M, Rezende de Castro R, Cuvelier G, Hamer I, Jadot M. A dileucine signal situated in the C-terminal tail of the lysosomal membrane protein p40 is responsible for its targeting to lysosomes. Biochem. J. 2008;414:431–440. - PubMed
-
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. β-arrestins and cell signaling. Annu. Rev. Physiol. 2007;69:483–510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

