Multiple roles for the first transmembrane domain of GABAA receptor subunits in neurosteroid modulation and spontaneous channel activity
- PMID: 20193738
- PMCID: PMC2927215
- DOI: 10.1016/j.neulet.2010.02.058
Multiple roles for the first transmembrane domain of GABAA receptor subunits in neurosteroid modulation and spontaneous channel activity
Abstract
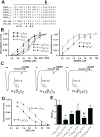
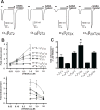
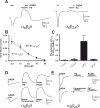
Neurosteroids exert potent physiological effects by allosterically modulating synaptic and extrasynaptic GABA(A) receptors. Some endogenous neurosteroids, such as 3alpha, 21-dihydroxy-5beta-pregnan-20-one (5alpha, 3alpha-THDOC), potentiate GABA(A) receptor function by interacting with a binding pocket defined by conserved residues in the first and fourth transmembrane (TM) domains of alpha subunits. Others, such as pregnenolone sulfate (PS), inhibit GABA(A) receptor function through as-yet unidentified binding sites. Here we investigate the mechanisms of PS inhibition of mammalian GABA(A) receptors, based on studies of PS inhibition of the UNC-49 GABA receptor, a GABA(A)-like receptor from Caenorhabditis elegans. In UNC-49, a 19 residue segment of TM1 can be mutated to increase or decrease PS sensitivity over a 20-fold range. Surprisingly, substituting these UNC-49 sequences into mammalian alpha(1), beta(2), and gamma(2) subunits did not produce the corresponding effects on PS sensitivity of the resulting chimeric receptors. Therefore, it is unlikely that a conserved PS binding pocket is formed at this site. However we observed several interesting unexpected effects. First, chimeric gamma2 subunits caused increased efficacy of 5alpha, 3alpha-THDOC potentiation; second, spontaneous gating of alpha(6)beta(2)delta receptors was blocked by PS, and reduced by chimeric beta(2) subunits; and third, direct activation of alpha(6)beta(2)delta receptors by 5alpha, 3alpha-THDOC was reduced by chimeric beta(2) subunits. These results reveal novel roles for non-alpha subunits in neurosteroid modulation and direct activation, and show that the beta subunit TM1 domain is important for spontaneous activity of extrasynaptic GABA(A) receptors.
Copyright (c) 2010 Elsevier Ireland Ltd. All rights reserved.
Figures

References
-
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–575. - PubMed
-
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. - PubMed
-
- Chang Y, Weiss DS. Substitutions of the highly conserved M2 leucine create spontaneously opening rho1 gamma-aminobutyric acid receptors. Mol Pharmacol. 1998;53:511–523. - PubMed
-
- Chen L, Cai W, Zhou R, Furuya K, Sokabe M. Modulatory metaplasticity induced by pregnenolone sulfate in the rat hippocampus: A leftward shift in LTP/LTD-frequency curve. Hippocampus. 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

