Regulation of base excision repair: Ntg1 nuclear and mitochondrial dynamic localization in response to genotoxic stress
- PMID: 20194111
- PMCID: PMC2896512
- DOI: 10.1093/nar/gkq108
Regulation of base excision repair: Ntg1 nuclear and mitochondrial dynamic localization in response to genotoxic stress
Abstract
Numerous human pathologies result from unrepaired oxidative DNA damage. Base excision repair (BER) is responsible for the repair of oxidative DNA damage that occurs in both nuclei and mitochondria. Despite the importance of BER in maintaining genomic stability, knowledge concerning the regulation of this evolutionarily conserved repair pathway is almost nonexistent. The Saccharomyces cerevisiae BER protein, Ntg1, relocalizes to organelles containing elevated oxidative DNA damage, indicating a novel mechanism of regulation for BER. We propose that dynamic localization of BER proteins is modulated by constituents of stress response pathways. In an effort to mechanistically define these regulatory components, the elements necessary for nuclear and mitochondrial localization of Ntg1 were identified, including a bipartite classical nuclear localization signal, a mitochondrial matrix targeting sequence and the classical nuclear protein import machinery. Our results define a major regulatory system for BER which when compromised, confers a mutator phenotype and sensitizes cells to the cytotoxic effects of DNA damage.
Figures
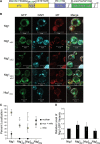

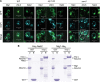

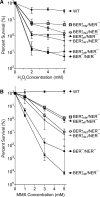
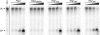

Similar articles
-
Dynamic compartmentalization of base excision repair proteins in response to nuclear and mitochondrial oxidative stress.Mol Cell Biol. 2009 Feb;29(3):794-807. doi: 10.1128/MCB.01357-08. Epub 2008 Nov 24. Mol Cell Biol. 2009. PMID: 19029246 Free PMC article.
-
Identification of SUMO modification sites in the base excision repair protein, Ntg1.DNA Repair (Amst). 2016 Dec;48:51-62. doi: 10.1016/j.dnarep.2016.10.011. Epub 2016 Oct 31. DNA Repair (Amst). 2016. PMID: 27839712 Free PMC article.
-
Different organization of base excision repair of uracil in DNA in nuclei and mitochondria and selective upregulation of mitochondrial uracil-DNA glycosylase after oxidative stress.Neuroscience. 2007 Apr 14;145(4):1201-12. doi: 10.1016/j.neuroscience.2006.10.010. Epub 2006 Nov 13. Neuroscience. 2007. PMID: 17101234
-
Regulation of intracellular localization of human MTH1, OGG1, and MYH proteins for repair of oxidative DNA damage.Prog Nucleic Acid Res Mol Biol. 2001;68:75-94. doi: 10.1016/s0079-6603(01)68091-7. Prog Nucleic Acid Res Mol Biol. 2001. PMID: 11554314 Review.
-
Enzymology of mitochondrial base excision repair.Prog Nucleic Acid Res Mol Biol. 2001;68:257-71. doi: 10.1016/s0079-6603(01)68105-4. Prog Nucleic Acid Res Mol Biol. 2001. PMID: 11554302 Review.
Cited by
-
DNA Repair and the Stability of the Plant Mitochondrial Genome.Int J Mol Sci. 2020 Jan 3;21(1):328. doi: 10.3390/ijms21010328. Int J Mol Sci. 2020. PMID: 31947741 Free PMC article. Review.
-
An Insight Into the Mechanism of Plant Organelle Genome Maintenance and Implications of Organelle Genome in Crop Improvement: An Update.Front Cell Dev Biol. 2021 Aug 10;9:671698. doi: 10.3389/fcell.2021.671698. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34447743 Free PMC article. Review.
-
Human NTHL1 expression and subcellular distribution determines cisplatin sensitivity in human lung epithelial and non-small cell lung cancer cells.NAR Cancer. 2024 Feb 21;6(1):zcae006. doi: 10.1093/narcan/zcae006. eCollection 2024 Mar. NAR Cancer. 2024. PMID: 38384388 Free PMC article.
-
The current state of eukaryotic DNA base damage and repair.Nucleic Acids Res. 2015 Dec 2;43(21):10083-101. doi: 10.1093/nar/gkv1136. Epub 2015 Oct 30. Nucleic Acids Res. 2015. PMID: 26519467 Free PMC article. Review.
-
Mitonuclear Interactions in the Maintenance of Mitochondrial Integrity.Life (Basel). 2020 Aug 31;10(9):173. doi: 10.3390/life10090173. Life (Basel). 2020. PMID: 32878185 Free PMC article. Review.
References
-
- Polyak K, Li Y, Zhu H, Lengauer C, Willson JK, Markowitz SD, Trush MA, Kinzler KW, Vogelstein B. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nat. Genet. 1998;20:291–293. - PubMed
-
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. - PubMed
-
- Altieri F, Grillo C, Maceroni M, Chichiarelli S. DNA damage and repair: from molecular mechanisms to health implications. Antioxid. Redox Signal. 2008;10:891–937. - PubMed
-
- Slupphaug G, Kavli B, Krokan HE. The interacting pathways for prevention and repair of oxidative DNA damage. Mutat. Res. 2003;531:231–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

