A mutation of the epithelial sodium channel associated with atypical cystic fibrosis increases channel open probability and reduces Na+ self inhibition
- PMID: 20194130
- PMCID: PMC2872728
- DOI: 10.1113/jphysiol.2009.180224
A mutation of the epithelial sodium channel associated with atypical cystic fibrosis increases channel open probability and reduces Na+ self inhibition
Abstract
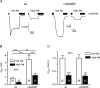
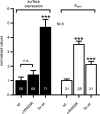
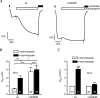
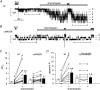
Increased activity of the epithelial sodium channel (ENaC) in the respiratory airways contributes to the pathophysiology of cystic fibrosis (CF), a genetic disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. In some patients suffering from atypical CF a mutation can be identified in only one CFTR allele. We recently identified in this group of CF patients a heterozygous mutation (W493R) in the alpha-subunit of ENaC. Here, we investigate the functional effects of this mutation by expressing wild-type alpha beta gamma ENaC or mutant alpha(W493R)beta gamma ENaC in Xenopus oocytes. The alpha W493R mutation stimulated amiloride-sensitive whole-cell currents (Delta I(ami)) by approximately 4-fold without altering the single-channel conductance or surface expression of ENaC. As these data suggest that the open probability (P(o)) of the mutant channel is increased, we investigated the proteolytic activation of ENaC by chymotrypsin. Single-channel recordings revealed that chymotrypsin activated near-silent channels in outside-out membrane patches from oocytes expressing wild-type ENaC, but not in membrane patches from oocytes expressing the mutant channel. In addition, the alpha W493R mutation abolished Na(+) self inhibition of ENaC, which might also contribute to its gain-of-function effects. We conclude that the alpha W493R mutation promotes constitutive activation of ENaC by reducing the inhibitory effect of extracellular Na(+) and decreasing the pool of near-silent channels. The resulting gain-of-function phenotype of the mutant channel might contribute to the pathophysiology of CF in patients carrying this mutation.
Figures
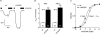


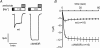
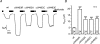
Similar articles
-
Functional characterization of a partial loss-of-function mutation of the epithelial sodium channel (ENaC) associated with atypical cystic fibrosis.Cell Physiol Biochem. 2010;25(1):145-58. doi: 10.1159/000272059. Epub 2009 Dec 22. Cell Physiol Biochem. 2010. PMID: 20054153
-
A mutation in the β-subunit of ENaC identified in a patient with cystic fibrosis-like symptoms has a gain-of-function effect.Am J Physiol Lung Cell Mol Physiol. 2013 Jan 1;304(1):L43-55. doi: 10.1152/ajplung.00093.2012. Epub 2012 Oct 19. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23087020
-
Effects of the serine/threonine kinase SGK1 on the epithelial Na(+) channel (ENaC) and CFTR: implications for cystic fibrosis.Cell Physiol Biochem. 2001;11(4):209-18. doi: 10.1159/000051935. Cell Physiol Biochem. 2001. PMID: 11509829
-
The epithelial sodium channel (ENaC) as a therapeutic target for cystic fibrosis lung disease.Expert Opin Ther Targets. 2018 Aug;22(8):687-701. doi: 10.1080/14728222.2018.1501361. Epub 2018 Jul 26. Expert Opin Ther Targets. 2018. PMID: 30028216 Review.
-
[CFTR and ENaC functions in cystic fibrosis].Medicina (B Aires). 2014;74(2):133-9. Medicina (B Aires). 2014. PMID: 24736260 Review. Spanish.
Cited by
-
Atomic force microscopy reveals the architecture of the epithelial sodium channel (ENaC).J Biol Chem. 2011 Sep 16;286(37):31944-52. doi: 10.1074/jbc.M111.275289. Epub 2011 Jul 20. J Biol Chem. 2011. PMID: 21775436 Free PMC article.
-
A Missense Mutation in the Extracellular Domain of αENaC Causes Liddle Syndrome.J Am Soc Nephrol. 2017 Nov;28(11):3291-3299. doi: 10.1681/ASN.2016111163. Epub 2017 Jul 14. J Am Soc Nephrol. 2017. PMID: 28710092 Free PMC article.
-
Epithelial Na+ Channel Regulation by Extracellular and Intracellular Factors.Annu Rev Physiol. 2018 Feb 10;80:263-281. doi: 10.1146/annurev-physiol-021317-121143. Epub 2017 Nov 9. Annu Rev Physiol. 2018. PMID: 29120692 Free PMC article. Review.
-
Neutrophil Elastase Activates Protease-activated Receptor-2 (PAR2) and Transient Receptor Potential Vanilloid 4 (TRPV4) to Cause Inflammation and Pain.J Biol Chem. 2015 May 29;290(22):13875-87. doi: 10.1074/jbc.M115.642736. Epub 2015 Apr 15. J Biol Chem. 2015. PMID: 25878251 Free PMC article.
-
Residues R282 and D341 act as electrostatic gates in the proton-dependent oligopeptide transporter PepT1.J Physiol. 2011 Feb 1;589(Pt 3):495-510. doi: 10.1113/jphysiol.2010.200469. Epub 2010 Nov 29. J Physiol. 2011. PMID: 21115649 Free PMC article.
References
-
- Azad AK, Rauh R, Vermeulen F, Jaspers M, Korbmacher J, Boissier B, et al. Mutations in the amiloride-sensitive epithelial sodium channel in patients with cystic fibrosis-like disease. Hum Mutat. 2009;30:1093–1103. - PubMed
-
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–158. - PubMed
-
- Boucher RC. Cystic fibrosis: a disease of vulnerability to airway surface dehydration. Trends Mol Med. 2007;13:231–240. - PubMed
-
- Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from theγ-subunit. J Biol Chem. 2007;282:6153–6160. - PubMed
-
- Caldwell RA, Boucher RC, Stutts MJ. Serine protease activation of near-silent epithelial Na+ channels. Am J Physiol Cell Physiol. 2004;286:C190–C194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

