A mutation of the epithelial sodium channel associated with atypical cystic fibrosis increases channel open probability and reduces Na+ self inhibition
- PMID: 20194130
- PMCID: PMC2872728
- DOI: 10.1113/jphysiol.2009.180224
A mutation of the epithelial sodium channel associated with atypical cystic fibrosis increases channel open probability and reduces Na+ self inhibition
Abstract
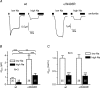
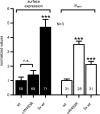
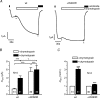
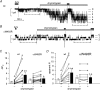
Increased activity of the epithelial sodium channel (ENaC) in the respiratory airways contributes to the pathophysiology of cystic fibrosis (CF), a genetic disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. In some patients suffering from atypical CF a mutation can be identified in only one CFTR allele. We recently identified in this group of CF patients a heterozygous mutation (W493R) in the alpha-subunit of ENaC. Here, we investigate the functional effects of this mutation by expressing wild-type alpha beta gamma ENaC or mutant alpha(W493R)beta gamma ENaC in Xenopus oocytes. The alpha W493R mutation stimulated amiloride-sensitive whole-cell currents (Delta I(ami)) by approximately 4-fold without altering the single-channel conductance or surface expression of ENaC. As these data suggest that the open probability (P(o)) of the mutant channel is increased, we investigated the proteolytic activation of ENaC by chymotrypsin. Single-channel recordings revealed that chymotrypsin activated near-silent channels in outside-out membrane patches from oocytes expressing wild-type ENaC, but not in membrane patches from oocytes expressing the mutant channel. In addition, the alpha W493R mutation abolished Na(+) self inhibition of ENaC, which might also contribute to its gain-of-function effects. We conclude that the alpha W493R mutation promotes constitutive activation of ENaC by reducing the inhibitory effect of extracellular Na(+) and decreasing the pool of near-silent channels. The resulting gain-of-function phenotype of the mutant channel might contribute to the pathophysiology of CF in patients carrying this mutation.
Figures
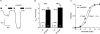


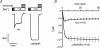
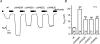
References
-
- Azad AK, Rauh R, Vermeulen F, Jaspers M, Korbmacher J, Boissier B, et al. Mutations in the amiloride-sensitive epithelial sodium channel in patients with cystic fibrosis-like disease. Hum Mutat. 2009;30:1093–1103. - PubMed
-
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–158. - PubMed
-
- Boucher RC. Cystic fibrosis: a disease of vulnerability to airway surface dehydration. Trends Mol Med. 2007;13:231–240. - PubMed
-
- Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from theγ-subunit. J Biol Chem. 2007;282:6153–6160. - PubMed
-
- Caldwell RA, Boucher RC, Stutts MJ. Serine protease activation of near-silent epithelial Na+ channels. Am J Physiol Cell Physiol. 2004;286:C190–C194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

