Multipotential differentiation of human anulus fibrosus cells: an in vitro study
- PMID: 20194326
- PMCID: PMC6882534
- DOI: 10.2106/JBJS.H.01672
Multipotential differentiation of human anulus fibrosus cells: an in vitro study
Abstract
Background: The existence of fibrocartilage, bone-like tissues, nerves, and blood vessels in the anulus fibrosus during intervertebral disc degeneration has been well documented. Migration of differentiated cells from outside the intervertebral disc has been hypothesized as a possible mechanism for the formation of these tissues. We hypothesized that the normal anulus fibrosus tissue contains multipotent progenitor cells, which are able to differentiate into cartilage and/or fibrocartilage cells, osteoblasts, neurons, and blood vessel cells.
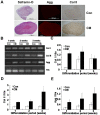
Methods: We isolated anulus fibrosus cells from the nondegenerative intervertebral discs of adolescent (thirteen to sixteen-year-old) patients with idiopathic scoliosis and cultured the cells in vitro in induction media containing different stimuli. Immunophenotypic analysis of cell surface markers was performed by flow cytometry. Expression of markers of adipogenesis, osteogenesis, chondrogenesis, neurogenesis, and differentiation into endothelial lineages was determined with use of immunostaining, cytohistological staining, and reverse transcription-polymerase chain reaction.
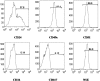
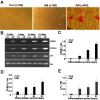
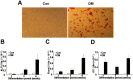
Results: Anulus fibrosus cells expressed several of the cell surface antigens that are sometimes associated with mesenchymal stem cells, including CD29, CD49e, CD51, CD73, CD90, CD105, CD166, CD184, and Stro-1, and two neuronal stem cell markers, nestin and neuron-specific enolase. Furthermore, varying the stimulants added to the induction media determined whether anulus fibrosus cells differentiated into adipocytes, osteoblasts, chondrocytes, neurons, or endothelial cells.
Conclusions: Anulus fibrosus cells isolated from nondegenerative intervertebral discs can differentiate into adipocytes, osteoblasts, chondrocytes, neurons, and endothelial cells in vitro.
Figures


References
-
- Diamond S Borenstein D. Chronic low back pain in a working-age adult. Best Pract Res Clin Rheumatol. 2006;20:707-20. - PubMed
-
- Luo X Pietrobon R Sun SX Liu GG Hey L. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine (Phila Pa 1976). 2004;29:79-86. - PubMed
-
- Battié MC Videman T Levalahti E Gill K Kaprio J. Heritability of low back pain and the role of disc degeneration. Pain. 2007;131:272-80. - PubMed
-
- Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581-5. - PubMed
-
- Walker MH Anderson DG. Molecular basis of intervertebral disc degeneration. Spine J. 2004;4:158S-66S. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

