A degron created by SMN2 exon 7 skipping is a principal contributor to spinal muscular atrophy severity
- PMID: 20194437
- PMCID: PMC2827839
- DOI: 10.1101/gad.1884910
A degron created by SMN2 exon 7 skipping is a principal contributor to spinal muscular atrophy severity
Abstract
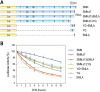
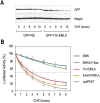
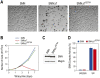
Spinal muscular atrophy (SMA) is caused by homozygous survival of motor neurons 1 (SMN1) gene deletions, leaving a duplicate gene, SMN2, as the sole source of SMN protein. However, most of the mRNA produced from SMN2 pre-mRNA is exon 7-skipped ( approximately 80%), resulting in a highly unstable and almost undetectable protein (SMNDelta7). We show that this splicing defect creates a potent degradation signal (degron; SMNDelta7-DEG) at SMNDelta7's C-terminal 15 amino acids. The S270A mutation inactivates SMNDelta7-DEG, generating a stable SMNDelta7 that rescues viability of SMN-deleted cells. These findings explain a key aspect of the SMA disease mechanism, and suggest new treatment approaches based on interference with SMNDelta7-DEG activity.
Figures
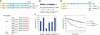
Similar articles
-
Advances in therapeutic development for spinal muscular atrophy.Future Med Chem. 2014 Jun;6(9):1081-99. doi: 10.4155/fmc.14.63. Future Med Chem. 2014. PMID: 25068989 Free PMC article. Review.
-
Hypoxia is a modifier of SMN2 splicing and disease severity in a severe SMA mouse model.Hum Mol Genet. 2012 Oct 1;21(19):4301-13. doi: 10.1093/hmg/dds263. Epub 2012 Jul 3. Hum Mol Genet. 2012. PMID: 22763238 Free PMC article.
-
hnRNP M facilitates exon 7 inclusion of SMN2 pre-mRNA in spinal muscular atrophy by targeting an enhancer on exon 7.Biochim Biophys Acta. 2014;1839(4):306-15. doi: 10.1016/j.bbagrm.2014.02.006. Epub 2014 Feb 15. Biochim Biophys Acta. 2014. PMID: 24533984
-
SMNDelta7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN.Hum Mol Genet. 2005 Mar 15;14(6):845-57. doi: 10.1093/hmg/ddi078. Epub 2005 Feb 9. Hum Mol Genet. 2005. PMID: 15703193
-
Mechanism of Splicing Regulation of Spinal Muscular Atrophy Genes.Adv Neurobiol. 2018;20:31-61. doi: 10.1007/978-3-319-89689-2_2. Adv Neurobiol. 2018. PMID: 29916015 Free PMC article. Review.
Cited by
-
Principles and correction of 5'-splice site selection.RNA Biol. 2022 Jan;19(1):943-960. doi: 10.1080/15476286.2022.2100971. RNA Biol. 2022. PMID: 35866748 Free PMC article. Review.
-
Molecular determinants of survival motor neuron (SMN) protein cleavage by the calcium-activated protease, calpain.PLoS One. 2010 Dec 30;5(12):e15769. doi: 10.1371/journal.pone.0015769. PLoS One. 2010. PMID: 21209906 Free PMC article.
-
Advances in therapeutic development for spinal muscular atrophy.Future Med Chem. 2014 Jun;6(9):1081-99. doi: 10.4155/fmc.14.63. Future Med Chem. 2014. PMID: 25068989 Free PMC article. Review.
-
High Concentration of an ISS-N1-Targeting Antisense Oligonucleotide Causes Massive Perturbation of the Transcriptome.Int J Mol Sci. 2021 Aug 4;22(16):8378. doi: 10.3390/ijms22168378. Int J Mol Sci. 2021. PMID: 34445083 Free PMC article.
-
Alternative Splicing Role in New Therapies of Spinal Muscular Atrophy.Genes (Basel). 2021 Aug 28;12(9):1346. doi: 10.3390/genes12091346. Genes (Basel). 2021. PMID: 34573328 Free PMC article. Review.
References
-
- Burnett BG, Crawford TO, Sumner CJ. Emerging treatment options for spinal muscular atrophy. Curr Treat Options Neurol. 2009a;11:90–101. - PubMed
-
- Chang HC, Hung WC, Chuang YJ, Jong YJ. Degradation of survival motor neuron (SMN) protein is mediated via the ubiquitin/proteasome pathway. Neurochem Int. 2004;45:1107–1112. - PubMed
-
- Cusco I, Barcelo MJ, Rojas-Garcia R, Illa I, Gamez J, Cervera C, Pou A, Izquierdo G, Baiget M, Tizzano EF. SMN2 copy number predicts acute or chronic spinal muscular atrophy but does not account for intrafamilial variability in siblings. J Neurol. 2006;253:21–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
