Human GLUD2 glutamate dehydrogenase is expressed in neural and testicular supporting cells
- PMID: 20194501
- PMCID: PMC2878061
- DOI: 10.1074/jbc.M109.092999
Human GLUD2 glutamate dehydrogenase is expressed in neural and testicular supporting cells
Abstract
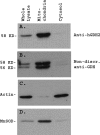
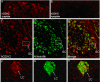
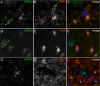
Mammalian glutamate dehydrogenase (GDH) is an allosterically regulated enzyme that is expressed widely. Its activity is potently inhibited by GTP and thought to be controlled by the need of the cell for ATP. In addition to this housekeeping human (h) GDH1, humans have acquired (via a duplication event) a highly homologous isoenzyme (hGDH2) that is resistant to GTP. Although transcripts of GLUD2, the gene encoding hGDH2, have been detected in human neural and testicular tissues, data on the endogenous protein are lacking. Here, we developed an antibody specific for hGDH2 and used it to study human tissues. Western blot analyses revealed, to our surprise, that endogenous hGDH2 is more densely expressed in testis than in brain. At the subcellular level, hGDH2 localized to mitochondria. Study of testicular tissue using immunocytochemical and immunofluorescence methods revealed that the Sertoli cells were strongly labeled by our anti-hGDH2 antibody. In human cerebral cortex, a robust labeling of astrocytes was detected, with neurons showing faint hGDH2 immunoreactivity. Astrocytes and Sertoli cells are known to support neurons and germ cells, respectively, providing them with lactate that largely derives from the tricarboxylic acid cycle via conversion of glutamate to alpha-ketoglutarate (GDH reaction). As hGDH2 is not subject to GTP control, the enzyme is able to metabolize glutamate even when the tricarboxylic acid cycle generates GTP amounts sufficient to inactivate the housekeeping hGDH1 protein. Hence, the selective expression of hGDH2 by astrocytes and Sertoli cells may provide a significant biological advantage by facilitating metabolic recycling processes essential to the supportive role of these cells.
Figures

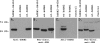




Similar articles
-
The human GLUD2 glutamate dehydrogenase and its regulation in health and disease.Neurochem Int. 2011 Sep;59(4):495-509. doi: 10.1016/j.neuint.2011.03.015. Epub 2011 Mar 21. Neurochem Int. 2011. PMID: 21420458 Review.
-
Widening Spectrum of Cellular and Subcellular Expression of Human GLUD1 and GLUD2 Glutamate Dehydrogenases Suggests Novel Functions.Neurochem Res. 2017 Jan;42(1):92-107. doi: 10.1007/s11064-016-1986-x. Epub 2016 Jul 16. Neurochem Res. 2017. PMID: 27422263
-
Expression of human GLUD2 glutamate dehydrogenase in human tissues: functional implications.Neurochem Int. 2012 Sep;61(4):455-62. doi: 10.1016/j.neuint.2012.06.007. Epub 2012 Jun 16. Neurochem Int. 2012. PMID: 22709674
-
Heterogeneous cellular distribution of glutamate dehydrogenase in brain and in non-neural tissues.Neurochem Res. 2014;39(3):500-15. doi: 10.1007/s11064-013-1235-5. Epub 2014 Jan 17. Neurochem Res. 2014. PMID: 24436052 Review.
-
The Glutamate Dehydrogenase Pathway and Its Roles in Cell and Tissue Biology in Health and Disease.Biology (Basel). 2017 Feb 8;6(1):11. doi: 10.3390/biology6010011. Biology (Basel). 2017. PMID: 28208702 Free PMC article. Review.
Cited by
-
Functional validation of a human GLUD2 variant in a murine model of Parkinson's disease.Cell Death Dis. 2020 Oct 22;11(10):897. doi: 10.1038/s41419-020-03043-2. Cell Death Dis. 2020. PMID: 33093440 Free PMC article.
-
Glutamate dehydrogenase hyperinsulinism: mechanisms, diagnosis, and treatment.Orphanet J Rare Dis. 2023 Jan 31;18(1):21. doi: 10.1186/s13023-023-02624-6. Orphanet J Rare Dis. 2023. PMID: 36721237 Free PMC article. Review.
-
Neuronal Glud1 (glutamate dehydrogenase 1) over-expressing mice: increased glutamate formation and synaptic release, loss of synaptic activity, and adaptive changes in genomic expression.Neurochem Int. 2011 Sep;59(4):473-81. doi: 10.1016/j.neuint.2011.03.003. Epub 2011 Mar 17. Neurochem Int. 2011. PMID: 21397652 Free PMC article.
-
Development of mice with brain-specific deletion of floxed glud1 (glutamate dehydrogenase 1) using cre recombinase driven by the nestin promoter.Neurochem Res. 2014;39(3):456-9. doi: 10.1007/s11064-013-1041-0. Epub 2013 Apr 18. Neurochem Res. 2014. PMID: 23595828 Review.
-
The role of glutamate dehydrogenase in the ageing brain.Front Pharmacol. 2025 Apr 28;16:1586655. doi: 10.3389/fphar.2025.1586655. eCollection 2025. Front Pharmacol. 2025. PMID: 40356954 Free PMC article. Review.
References
-
- Smith E. (1979) Proc. Am. Philos. Soc. 123, 73–84
-
- Hudson R. C., Daniel R. M. (1993) Comp. Biochem. Physiol. B 106, 767–792 - PubMed
-
- Plaitakis A., Berl S., Yahr M. D. (1984) Ann. Neurol. 15, 144–153 - PubMed
-
- Shashidharan P., Michaelidis T. M., Robakis N. K., Kresovali A., Papamatheakis J., Plaitakis A. (1994) J. Biol. Chem. 269, 16971–16976 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

