Formation of a cyclopropyl epoxide via a leukotriene A synthase-related pathway in an anaerobic reaction of soybean lipoxygenase-1 with 15S-hydroperoxyeicosatetraenoic acid: evidence that oxygen access is a determinant of secondary reactions with fatty acid hydroperoxides
- PMID: 20194505
- PMCID: PMC2859502
- DOI: 10.1074/jbc.M109.084632
Formation of a cyclopropyl epoxide via a leukotriene A synthase-related pathway in an anaerobic reaction of soybean lipoxygenase-1 with 15S-hydroperoxyeicosatetraenoic acid: evidence that oxygen access is a determinant of secondary reactions with fatty acid hydroperoxides
Abstract
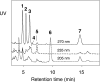
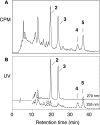
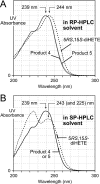
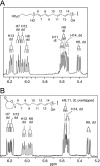
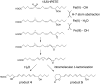
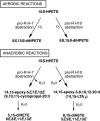
The further conversion of an arachidonic acid hydroperoxide to a leukotriene A (LTA) type epoxide by specific lipoxygenase (LOX) enzymes constitutes a key step in inflammatory mediator biosynthesis. Whereas mammalian 5-LOX transforms its primary product (5S-hydroperoxyeicosatetraenoic acid; 5S-HPETE) almost exclusively to LTA(4), the model enzyme, soybean LOX-1, normally produces no detectable leukotrienes and instead further oxygenates its primary product 15S-HPETE to 5,15- and 8,15-dihydroperoxides. Mammalian 15-LOX-1 displays both types of activity. We reasoned that availability of molecular oxygen within the LOX active site favors oxygenation, whereas lack of O(2) promotes LTA epoxide synthesis. To test this, we reacted 15S-HPETE with soybean LOX-1 under anaerobic conditions and identified the products by high pressure liquid chromatography, UV, mass spectrometry, and NMR. Among the products, we identified a pair of 8,15-dihydroxy diastereomers with all-trans-conjugated trienes that incorporated (18)O from H(2)(18)O at C-8, indicative of the formation of 14,15-LTA(4). A pair of 5,15-dihydroxy diastereomers containing two trans,trans-conjugated dienes (6E,8E,11E,13E) and that incorporated (18)O from H(2)(18)O at C-5 was deduced to arise from hydrolysis of a novel epoxide containing a cyclopropyl ring, 14,15-epoxy-[9,10,11-cyclopropyl]-eicosa-5Z,7E,13E-trienoic acid. Also identified was the delta-lactone of the 5,15-diol, a derivative that exhibited no (18)O incorporation due to its formation by intramolecular reaction of the carboxyl anion with the proposed epoxide intermediate. Our results support a model in which access to molecular oxygen within the active site directs the outcome from competing pathways in the secondary reactions of lipoxygenases.
Figures

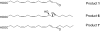

Similar articles
-
Biosynthesis, isolation, and NMR analysis of leukotriene A epoxides: substrate chirality as a determinant of the cis or trans epoxide configuration.J Lipid Res. 2013 Mar;54(3):754-761. doi: 10.1194/jlr.M033746. Epub 2012 Dec 13. J Lipid Res. 2013. PMID: 23242647 Free PMC article.
-
On the relationships of substrate orientation, hydrogen abstraction, and product stereochemistry in single and double dioxygenations by soybean lipoxygenase-1 and its Ala542Gly mutant.J Biol Chem. 2005 Nov 18;280(46):38756-66. doi: 10.1074/jbc.M504870200. Epub 2005 Sep 12. J Biol Chem. 2005. PMID: 16157595 Free PMC article.
-
Cytochrome P450-dependent transformations of 15R- and 15S-hydroperoxyeicosatetraenoic acids: stereoselective formation of epoxy alcohol products.Biochemistry. 1996 Jan 16;35(2):464-71. doi: 10.1021/bi952081v. Biochemistry. 1996. PMID: 8555216
-
The eosinophil chemoattractant 5-oxo-ETE and the OXE receptor.Prog Lipid Res. 2013 Oct;52(4):651-65. doi: 10.1016/j.plipres.2013.09.001. Epub 2013 Sep 19. Prog Lipid Res. 2013. PMID: 24056189 Free PMC article. Review.
-
Stereochemical aspects of fatty acid oxidation: hydroperoxide isomerases.Acta Chem Scand (Cph). 1996 Mar;50(3):219-24. doi: 10.3891/acta.chem.scand.50-0219. Acta Chem Scand (Cph). 1996. PMID: 8901175 Review.
Cited by
-
Demonstration of HNE-related aldehyde formation via lipoxygenase-catalyzed synthesis of a bis-allylic dihydroperoxide intermediate.Chem Res Toxicol. 2013 Jun 17;26(6):896-903. doi: 10.1021/tx4000396. Epub 2013 May 23. Chem Res Toxicol. 2013. PMID: 23668325 Free PMC article.
-
Applications of stereospecifically-labeled Fatty acids in oxygenase and desaturase biochemistry.Lipids. 2012 Feb;47(2):101-16. doi: 10.1007/s11745-011-3612-7. Epub 2011 Oct 5. Lipids. 2012. PMID: 21971646 Free PMC article. Review.
-
12R-lipoxygenase activity is reduced in photodamaged facial stratum corneum. A novel activity assay indicates a key function in corneocyte maturation.Int J Cosmet Sci. 2019 Jun;41(3):274-280. doi: 10.1111/ics.12532. Epub 2019 May 28. Int J Cosmet Sci. 2019. PMID: 30993698 Free PMC article.
-
On the role of molecular oxygen in lipoxygenase activation: comparison and contrast of epidermal lipoxygenase-3 with soybean lipoxygenase-1.J Biol Chem. 2010 Dec 17;285(51):39876-87. doi: 10.1074/jbc.M110.180794. Epub 2010 Oct 5. J Biol Chem. 2010. PMID: 20923767 Free PMC article.
-
Thioperamide treats neonatal hypoxic-ischemic encephalopathy by postsynaptic H1 receptors.Neural Regen Res. 2013 Jul 5;8(19):1814-22. doi: 10.3969/j.issn.1673-5374.2013.19.009. Neural Regen Res. 2013. PMID: 25206478 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

