Evolution and multifarious horizontal transfer of an alternative biosynthetic pathway for the alternative polyamine sym-homospermidine
- PMID: 20194510
- PMCID: PMC2863184
- DOI: 10.1074/jbc.M110.107219
Evolution and multifarious horizontal transfer of an alternative biosynthetic pathway for the alternative polyamine sym-homospermidine
Abstract
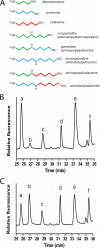
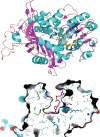
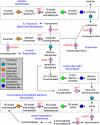
Polyamines are small flexible organic polycations found in almost all cells. They likely existed in the last universal common ancestor of all extant life, and yet relatively little is understood about their biological function, especially in bacteria and archaea. Unlike eukaryotes, where the predominant polyamine is spermidine, bacteria may contain instead an alternative polyamine, sym-homospermidine. We demonstrate that homospermidine synthase (HSS) has evolved vertically, primarily in the alpha-Proteobacteria, but enzymatically active, diverse HSS orthologues have spread by horizontal gene transfer to other bacteria, bacteriophage, archaea, eukaryotes, and viruses. By expressing diverse HSS orthologues in Escherichia coli, we demonstrate in vivo the production of co-products diaminopropane and N(1)-aminobutylcadaverine, in addition to sym-homospermidine. We show that sym-homospermidine is required for normal growth of the alpha-proteobacterium Rhizobium leguminosarum. However, sym-homospermidine can be replaced, for growth restoration, by the structural analogues spermidine and sym-norspermidine, suggesting that the symmetrical or unsymmetrical form and carbon backbone length are not critical for polyamine function in growth. We found that the HSS enzyme evolved from the alternative spermidine biosynthetic pathway enzyme carboxyspermidine dehydrogenase. The structure of HSS is related to lysine metabolic enzymes, and HSS and carboxyspermidine dehydrogenase evolved from the aspartate family of pathways. Finally, we show that other bacterial phyla such as Cyanobacteria and some alpha-Proteobacteria synthesize sym-homospermidine by an HSS-independent pathway, very probably based on deoxyhypusine synthase orthologues, similar to the alternative homospermidine synthase found in some plants. Thus, bacteria can contain alternative biosynthetic pathways for both spermidine and sym-norspermidine and distinct alternative pathways for sym-homospermidine.
Figures





Similar articles
-
Alternative spermidine biosynthetic route is critical for growth of Campylobacter jejuni and is the dominant polyamine pathway in human gut microbiota.J Biol Chem. 2011 Dec 16;286(50):43301-12. doi: 10.1074/jbc.M111.307835. Epub 2011 Oct 24. J Biol Chem. 2011. PMID: 22025614 Free PMC article.
-
Different polyamine pathways from bacteria have replaced eukaryotic spermidine biosynthesis in ciliates Tetrahymena thermophila and Paramecium tetaurelia.Mol Microbiol. 2015 Sep;97(5):791-807. doi: 10.1111/mmi.13066. Epub 2015 Jun 8. Mol Microbiol. 2015. PMID: 25994085
-
An alternative polyamine biosynthetic pathway is widespread in bacteria and essential for biofilm formation in Vibrio cholerae.J Biol Chem. 2009 Apr 10;284(15):9899-907. doi: 10.1074/jbc.M900110200. Epub 2009 Feb 5. J Biol Chem. 2009. PMID: 19196710 Free PMC article.
-
Spermidine metabolism in parasitic protozoa--a comparison to the situation in prokaryotes, viruses, plants and fungi.Folia Parasitol (Praha). 2003 Mar;50(1):3-18. doi: 10.14411/fp.2003.002. Folia Parasitol (Praha). 2003. PMID: 12735718 Review.
-
Polyamine function in archaea and bacteria.J Biol Chem. 2018 Nov 30;293(48):18693-18701. doi: 10.1074/jbc.TM118.005670. Epub 2018 Sep 25. J Biol Chem. 2018. PMID: 30254075 Free PMC article. Review.
Cited by
-
New aspect of plant-rhizobia interaction: alkaloid biosynthesis in Crotalaria depends on nodulation.Proc Natl Acad Sci U S A. 2015 Mar 31;112(13):4164-9. doi: 10.1073/pnas.1423457112. Epub 2015 Mar 16. Proc Natl Acad Sci U S A. 2015. PMID: 25775562 Free PMC article.
-
Comprehensive Structural Characterization of the Bacterial Homospermidine Synthase-an Essential Enzyme of the Polyamine Metabolism.Sci Rep. 2016 Jan 18;6:19501. doi: 10.1038/srep19501. Sci Rep. 2016. PMID: 26776105 Free PMC article.
-
The Essential Role of Spermidine in Growth of Agrobacterium tumefaciens Is Determined by the 1,3-Diaminopropane Moiety.ACS Chem Biol. 2016 Feb 19;11(2):491-9. doi: 10.1021/acschembio.5b00893. Epub 2015 Dec 28. ACS Chem Biol. 2016. PMID: 26682642 Free PMC article.
-
Independent evolutionary origins of functional polyamine biosynthetic enzyme fusions catalysing de novo diamine to triamine formation.Mol Microbiol. 2011 Aug;81(4):1109-24. doi: 10.1111/j.1365-2958.2011.07757.x. Epub 2011 Jul 18. Mol Microbiol. 2011. PMID: 21762220 Free PMC article.
-
The Biosynthesis and Functions of Polyamines in the Interaction of Plant Growth-Promoting Rhizobacteria with Plants.Plants (Basel). 2023 Jul 17;12(14):2671. doi: 10.3390/plants12142671. Plants (Basel). 2023. PMID: 37514285 Free PMC article. Review.
References
-
- Cohen S. S. (1998) A Guide to the Polyamines, Oxford University Press, New York, NY
-
- Chattopadhyay M. K., Murakami Y., Matsufuji S. (2001) J. Biol. Chem. 276, 21235–21241 - PubMed
-
- Jin Y., Bok J. W., Guzman-de-Peña D., Keller N. P. (2002) Mol. Microbiol. 46, 801–812 - PubMed
-
- Guevara-Olvera L., Xoconostle-Cázares B., Ruiz-Herrera J. (1997) Microbiology 143, 2237–2245 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

