Asymmetric acetylation of the cyclooxygenase-2 homodimer by aspirin and its effects on the oxygenation of arachidonic, eicosapentaenoic, and docosahexaenoic acids
- PMID: 20194532
- PMCID: PMC2879920
- DOI: 10.1124/mol.109.063115
Asymmetric acetylation of the cyclooxygenase-2 homodimer by aspirin and its effects on the oxygenation of arachidonic, eicosapentaenoic, and docosahexaenoic acids
Abstract
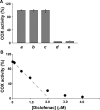
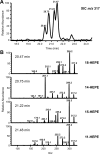
Prostaglandin endoperoxide H synthases (PGHS)-1 and -2, also called cyclooxygenases, convert arachidonic acid (AA) to prostaglandin H(2) (PGH(2)) in the committed step of prostaglandin biosynthesis. Both enzymes are homodimers, but the monomers often behave asymmetrically as conformational heterodimers during catalysis and inhibition. Here we report that aspirin maximally acetylates one monomer of human (hu) PGHS-2. The acetylated monomer of aspirin-treated huPGHS-2 forms 15-hydroperoxyeicosatetraenoic acid from AA, whereas the nonacetylated partner monomer forms mainly PGH(2) but only at 15 to 20% of the rate of native huPGHS-2. These latter conclusions are based on the findings that the nonsteroidal anti-inflammatory drug diclofenac binds a single monomer of native huPGHS-2, having an unmodified Ser530 to inhibit the enzyme, and that diclofenac inhibits PGH(2) but not 15-hydroperoxyeicosatraenoic acid formation by acetylated huPGHS-2. The 18R- and 17R-resolvins putatively involved in resolution of inflammation are reportedly formed via aspirin-acetylated PGHS-2 from eicosapentaenoic acid and docosahexaenoic acid, respectively, so we also characterized the oxygenation of these omega-3 fatty acids by aspirin-treated huPGHS-2. Our in vitro studies suggest that 18R- and 17R-resolvins could be formed only at low rates corresponding to less than 1 and 5%, respectively, of the rates of formation of PGH(2) by native PGHS-2.
Figures


References
-
- Bhattacharyya DK, Lecomte M, Rieke CJ, Garavito M, Smith WL. (1996) Involvement of arginine 120, glutamate 524, and tyrosine 355 in the binding of arachidonate and 2-phenylpropionic acid inhibitors to the cyclooxygenase active site of ovine prostaglandin endoperoxide H synthase-1. J Biol Chem 271:2179–2184 - PubMed
-
- Blobaum AL, Marnett LJ. (2007) Structural and functional basis of cyclooxygenase inhibition. J Med Chem 50:1425–1441 - PubMed
-
- DeWitt DL. (1999) Cox-2-selective inhibitors: the new super aspirins. Mol Pharmacol 55:625–631 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

