Antigen-presenting dendritic cells rescue CD4-depleted CCR2-/- mice from lethal Histoplasma capsulatum infection
- PMID: 20194586
- PMCID: PMC2863525
- DOI: 10.1128/IAI.00065-10
Antigen-presenting dendritic cells rescue CD4-depleted CCR2-/- mice from lethal Histoplasma capsulatum infection
Abstract
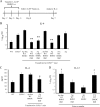
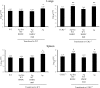
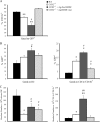
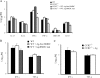
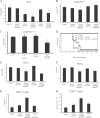
Excessive production of interleukin-4 impairs clearance of the fungal pathogen Histoplasma capsulatum in mice lacking the chemokine receptor CCR2. An increase in the interleukin-4 level is associated with decreased recruitment of dendritic cells to lungs; therefore, we investigated the possibility that these cells influence interleukin-4 production. Adoptive transfer of wild-type or CCR2(-/-) bone marrow-derived dendritic cells loaded with heat-killed yeast cells to infected CCR2(-/-) mice suppressed interleukin-4 transcription. Surprisingly, transfer of cells did not reduce the fungal burden despite the fact that it limited interleukin-4 transcription. Yeast cell-loaded bone marrow-derived dendritic cell-mediated regulation of interleukin-4 transcription was dependent on major histocompatibility complex II antigen presentation to CD4(+) T cells. We previously showed that CD4(+) T cells were a source of interleukin-4 in infected CCR2(-/-) mice, but their contribution to the TH2 phenotype was unclear. Here we demonstrated that these cells were functionally important since elimination of them prior to infection, but not elimination of them at the time of infection, reduced the interleukin-4 level in infected CCR2(-/-) mice. However, the fungal burden was reduced only in CD4-depleted CCR2(-/-) mice that received yeast cell-loaded bone marrow-derived dendritic cells. Taken together, the data indicate that generation of excess interleukin-4 in lungs of H. capsulatum-infected CCR2(-/-) mice is at least partially a consequence of decreased recruitment of dendritic cells capable of antigen presentation. Furthermore, CD4(+) T cells had a deleterious impact on immunity in infected CCR2(-/-) mice.
Figures
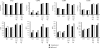

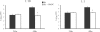
Similar articles
-
MyD88-dependent signaling drives host survival and early cytokine production during Histoplasma capsulatum infection.Infect Immun. 2015 Apr;83(4):1265-75. doi: 10.1128/IAI.02619-14. Epub 2015 Jan 12. Infect Immun. 2015. PMID: 25583527 Free PMC article.
-
The CCL7-CCL2-CCR2 axis regulates IL-4 production in lungs and fungal immunity.J Immunol. 2009 Aug 1;183(3):1964-74. doi: 10.4049/jimmunol.0901316. Epub 2009 Jul 8. J Immunol. 2009. PMID: 19587014 Free PMC article.
-
Dendritic cells cross-present exogenous fungal antigens to stimulate a protective CD8 T cell response in infection by Histoplasma capsulatum.J Immunol. 2005 May 15;174(10):6282-91. doi: 10.4049/jimmunol.174.10.6282. J Immunol. 2005. PMID: 15879127
-
Resistance mechanisms in murine experimental histoplasmosis.Arch Med Res. 1993 Autumn;24(3):233-8. Arch Med Res. 1993. PMID: 7905306 Review.
-
Host response and Histoplasma capsulatum/macrophage molecular interactions.Med Mycol. 2000 Dec;38(6):399-406. doi: 10.1080/mmy.38.6.399.406. Med Mycol. 2000. PMID: 11204877 Review.
Cited by
-
Key thermally dimorphic fungal pathogens: shaping host immunity.Open Biol. 2022 Mar;12(3):210219. doi: 10.1098/rsob.210219. Epub 2022 Mar 9. Open Biol. 2022. PMID: 35259948 Free PMC article. Review.
-
First Line of Defense: Innate Cell-Mediated Control of Pulmonary Aspergillosis.Front Microbiol. 2016 Mar 3;7:272. doi: 10.3389/fmicb.2016.00272. eCollection 2016. Front Microbiol. 2016. PMID: 26973640 Free PMC article. Review.
-
Mononuclear phagocyte-mediated antifungal immunity: the role of chemotactic receptors and ligands.Cell Mol Life Sci. 2015 Jun;72(11):2157-75. doi: 10.1007/s00018-015-1858-6. Epub 2015 Feb 26. Cell Mol Life Sci. 2015. PMID: 25715741 Free PMC article. Review.
-
Dendritic cell interactions with Histoplasma and Paracoccidioides.Virulence. 2015;6(5):424-32. doi: 10.4161/21505594.2014.965586. Epub 2015 May 1. Virulence. 2015. PMID: 25933034 Free PMC article. Review.
-
Deciphering the pathways of death of Histoplasma capsulatum-infected macrophages: implications for the immunopathogenesis of early infection.J Immunol. 2012 Jan 1;188(1):334-44. doi: 10.4049/jimmunol.1102175. Epub 2011 Nov 18. J Immunol. 2012. PMID: 22102723 Free PMC article.
References
-
- Aldridge, J. R., Jr., C. E. Moseley, D. A. Boltz, N. J. Negovetich, C. Reynolds, J. Franks, S. A. Brown, P. C. Doherty, R. G. Webster, and P. G. Thomas. 2009. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc. Natl. Acad. Sci. U. S. A. 106:5306-5311. - PMC - PubMed
-
- Allendoerfer, R., and G. S. Deepe, Jr. 1998. Blockade of endogenous TNF-α exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J. Immunol. 160:6072-6082. - PubMed
-
- Allendoerfer, R., and G. S. Deepe, Jr. 2000. Regulation of infection with Histoplasma capsulatum by TNFR1 and -2. J. Immunol. 165:2657-2664. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

