Indispensable role for the eukaryotic-like ankyrin domains of the ankyrin B effector of Legionella pneumophila within macrophages and amoebae
- PMID: 20194593
- PMCID: PMC2863551
- DOI: 10.1128/IAI.01450-09
Indispensable role for the eukaryotic-like ankyrin domains of the ankyrin B effector of Legionella pneumophila within macrophages and amoebae
Erratum in
- Infect Immun. 2013 Jul;81(7):2660
Abstract
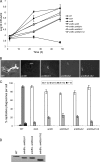
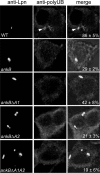
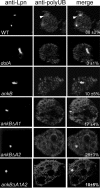
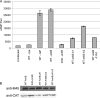
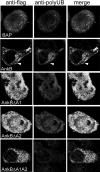
The Dot/Icm-translocated ankyrin B (AnkB) effector of Legionella pneumophila exhibits molecular mimicry of eukaryotic F-box proteins and is essential for intracellular replication in macrophages and protozoa. In addition to two eukaryotic-like ankyrin (ANK) domains, AnkB harbors a conserved eukaryotic F-box domain, which is involved in polyubiquitination of proteins throughout the eukaryotic kingdom. We have recently shown that the F-box domain of the AnkB effector is essential for decoration of the Legionella-containing vacuole (LCV) with polyubiquitinated proteins within macrophages and protozoan hosts. To decipher the role of the two ANK domains in the function of AnkB, we have constructed in-frame deletion of either or both of the ANK domain-encoding regions (ankB Delta A1, ankB Delta A2, and ankB Delta A1A2) to trans-complement the ankB null mutant. Deletion of the ANK domains results in defects in intracellular proliferation and decoration of the LCV with polyubiquitinated proteins. Export of the truncated variants of AnkB was reduced, and this may account for the observed defects. However, while full-length AnkB ectopically expressed in mammalian cells trans-rescues the ankB null mutant for intracellular proliferation, ectopic expression of AnkB Delta A1, AnkB Delta A2, and AnkB Delta A1A2 fails to trans-rescue the ankB null mutant. Importantly, ectopically expressed full-length AnkB is targeted to the host cell plasma membrane, where it recruits polyubiquitinated proteins. In contrast, AnkB Delta A1, AnkB Delta A2, and AnkB Delta A1A2 are diffusely distributed throughout the cytosol and fail to recruit polyubiquitinated proteins. We conclude that the two eukaryotic-like ANK domains of AnkB are essential for intracellular proliferation, for targeting AnkB to the host membranes, and for decoration of the LCV with polyubiquitinated proteins.
Figures



Similar articles
-
Molecular mimicry by an F-box effector of Legionella pneumophila hijacks a conserved polyubiquitination machinery within macrophages and protozoa.PLoS Pathog. 2009 Dec;5(12):e1000704. doi: 10.1371/journal.ppat.1000704. Epub 2009 Dec 24. PLoS Pathog. 2009. PMID: 20041211 Free PMC article.
-
Molecular Characterization of Exploitation of the Polyubiquitination and Farnesylation Machineries of Dictyostelium Discoideum by the AnkB F-Box Effector of Legionella Pneumophila.Front Microbiol. 2011 Feb 14;2:23. doi: 10.3389/fmicb.2011.00023. eCollection 2011. Front Microbiol. 2011. PMID: 21687415 Free PMC article.
-
A Dot/Icm-translocated ankyrin protein of Legionella pneumophila is required for intracellular proliferation within human macrophages and protozoa.Mol Microbiol. 2008 Nov;70(4):908-23. doi: 10.1111/j.1365-2958.2008.06453.x. Epub 2008 Sep 22. Mol Microbiol. 2008. PMID: 18811729 Free PMC article.
-
Cellular microbiology and molecular ecology of Legionella-amoeba interaction.Virulence. 2013 May 15;4(4):307-14. doi: 10.4161/viru.24290. Epub 2013 Mar 27. Virulence. 2013. PMID: 23535283 Free PMC article. Review.
-
Amoeba host-Legionella synchronization of amino acid auxotrophy and its role in bacterial adaptation and pathogenic evolution.Environ Microbiol. 2014 Feb;16(2):350-8. doi: 10.1111/1462-2920.12290. Epub 2013 Oct 21. Environ Microbiol. 2014. PMID: 24112119 Free PMC article. Review.
Cited by
-
The Functional Differences between the GroEL Chaperonin of Escherichia coli and the HtpB Chaperonin of Legionella pneumophila Can Be Mapped to Specific Amino Acid Residues.Biomolecules. 2021 Dec 31;12(1):59. doi: 10.3390/biom12010059. Biomolecules. 2021. PMID: 35053207 Free PMC article.
-
Secreted Effectors Encoded within and outside of the Francisella Pathogenicity Island Promote Intramacrophage Growth.Cell Host Microbe. 2016 Nov 9;20(5):573-583. doi: 10.1016/j.chom.2016.10.008. Cell Host Microbe. 2016. PMID: 27832588 Free PMC article.
-
Genomic insights into the marine sponge microbiome.Nat Rev Microbiol. 2012 Sep;10(9):641-54. doi: 10.1038/nrmicro2839. Epub 2012 Jul 30. Nat Rev Microbiol. 2012. PMID: 22842661 Review.
-
Orientia tsutsugamushi uses two Ank effectors to modulate NF-κB p65 nuclear transport and inhibit NF-κB transcriptional activation.PLoS Pathog. 2018 May 7;14(5):e1007023. doi: 10.1371/journal.ppat.1007023. eCollection 2018 May. PLoS Pathog. 2018. PMID: 29734393 Free PMC article.
-
Bacterial Type IV secretion systems: versatile virulence machines.Future Microbiol. 2012 Feb;7(2):241-57. doi: 10.2217/fmb.11.150. Future Microbiol. 2012. PMID: 22324993 Free PMC article. Review.
References
-
- Al-Khodor, S., T. Al-Quadan, and Y. Abu Kwaik. Temporal and differential regulation of expression of the eukaryotic-like ankyrin effectors of L. pneumophila. Environ. Microbiol., in press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

