Role of host sphingosine kinase 1 in the lung response against Cryptococcosis
- PMID: 20194596
- PMCID: PMC2863500
- DOI: 10.1128/IAI.01140-09
Role of host sphingosine kinase 1 in the lung response against Cryptococcosis
Abstract
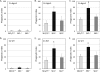
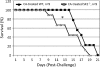
Cryptococcus neoformans is a fungal pathogen causing pulmonary infection and a life-threatening meningoencephalitis in human hosts. The fungus infects the host through inhalation, and thus, the host response in the lung environment is crucial for containment or dissemination of C. neoformans to other organs. In the lung, alveolar macrophages (AMs) are key players in the host lung immune response, and upon phagocytosis, they can kill C. neoformans by evoking an effective immune response through a variety of signaling molecules. On the other hand, under conditions not yet fully defined, the fungus is able to survive and proliferate within macrophages. Since the host sphingosine kinase 1 (SK1) regulates many signaling functions of immune cells, particularly in macrophages, in this study we determined the role of SK1 in the host response to C. neoformans infection. Using wild-type (SK1/2(+/+)) and SK1-deficient (SK1(-/-)) mice, we found that SK1 is dispensable during infection with a facultative intracellular wild-type C. neoformans strain. However, SK1 is required to form a host lung granuloma and to prevent brain infection by a C. neoformans mutant strain lacking the cell wall-associated glycosphingolipid glucosylceramide (Delta gcs1), previously characterized as a mutant able to replicate only intracellularly. Specifically, in contrast to those from SK1/2(+/+) mice, lungs from SK1(-/-) mice have no collagen deposition upon infection with C. neoformans Delta gcs1, and AMs from these mice contain significantly more C. neoformans cells than AMs from SK1/2(+/+) mice, suggesting that under conditions in which C. neoformans is more internalized by AMs, SK1 may become important to control C. neoformans infection. Indeed, when we induced immunosuppression, a host condition in which wild-type C. neoformans cells are increasingly found intracellularly, SK1(-/-) survived significantly less than SK1/2(+/+) mice infected with a facultative intracellular wild-type strain, suggesting that SK1 has an important role in controlling C. neoformans infection under conditions in which the fungus is predominantly found intracellularly.
Figures




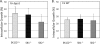

Similar articles
-
The Granuloma Response Controlling Cryptococcosis in Mice Depends on the Sphingosine Kinase 1-Sphingosine 1-Phosphate Pathway.Infect Immun. 2015 Jul;83(7):2705-13. doi: 10.1128/IAI.00056-15. Epub 2015 Apr 20. Infect Immun. 2015. PMID: 25895971 Free PMC article.
-
Role of sphingosine-1-phosphate (S1P) and S1P receptor 2 in the phagocytosis of Cryptococcus neoformans by alveolar macrophages.Microbiology (Reading). 2011 May;157(Pt 5):1416-1427. doi: 10.1099/mic.0.045989-0. Epub 2011 Feb 3. Microbiology (Reading). 2011. PMID: 21292747 Free PMC article.
-
Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice.Infect Immun. 2007 Oct;75(10):4792-8. doi: 10.1128/IAI.00587-07. Epub 2007 Jul 30. Infect Immun. 2007. PMID: 17664261 Free PMC article.
-
Catch me if you can: phagocytosis and killing avoidance by Cryptococcus neoformans.FEMS Immunol Med Microbiol. 2012 Mar;64(2):147-61. doi: 10.1111/j.1574-695X.2011.00871.x. FEMS Immunol Med Microbiol. 2012. PMID: 22029633 Review.
-
Paradoxical roles of alveolar macrophages in the host response to Cryptococcus neoformans.J Infect Chemother. 2012 Feb;18(1):1-9. doi: 10.1007/s10156-011-0306-2. Epub 2011 Nov 2. J Infect Chemother. 2012. PMID: 22045161 Free PMC article. Review.
Cited by
-
PET Study of Sphingosine-1-phosphate Receptor 1 Expression in Response to S. aureus Infection.Mol Imaging. 2021 Oct 4;2021:9982020. doi: 10.1155/2021/9982020. eCollection 2021. Mol Imaging. 2021. PMID: 34934406 Free PMC article.
-
Francisella tularensis LVS induction of prostaglandin biosynthesis by infected macrophages requires specific host phospholipases and lipid phosphatases.Infect Immun. 2014 Aug;82(8):3299-311. doi: 10.1128/IAI.02060-14. Epub 2014 May 27. Infect Immun. 2014. PMID: 24866789 Free PMC article.
-
Sphingolipids as targets for treatment of fungal infections.Future Med Chem. 2016 Aug;8(12):1469-84. doi: 10.4155/fmc-2016-0053. Epub 2016 Aug 9. Future Med Chem. 2016. PMID: 27502288 Free PMC article. Review.
-
An Immunogenic and Slow-Growing Cryptococcal Strain Induces a Chronic Granulomatous Infection in Murine Lungs.Infect Immun. 2022 Jun 16;90(6):e0058021. doi: 10.1128/iai.00580-21. Epub 2022 May 19. Infect Immun. 2022. PMID: 35587201 Free PMC article.
-
FTY720 reactivates cryptococcal granulomas in mice through S1P receptor 3 on macrophages.J Clin Invest. 2020 Sep 1;130(9):4546-4560. doi: 10.1172/JCI136068. J Clin Invest. 2020. PMID: 32484801 Free PMC article.
References
-
- Allende, M. L., T. Sasaki, H. Kawai, A. Olivera, Y. Mi, G. van Echten-Deckert, R. Hajdu, M. Rosenbach, C. A. Keohane, S. Mandala, S. Spiegel, and R. L. Proia. 2004. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 279:52487-52492. - PubMed
-
- Ammit, A. J., A. T. Hastie, L. C. Edsall, R. K. Hoffman, Y. Amrani, V. P. Krymskaya, S. A. Kane, S. P. Peters, R. B. Penn, S. Spiegel, and R. A. Panettieri, Jr. 2001. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB J. 15:1212-1214. - PubMed
-
- Billich, A., F. Bornancin, P. Devay, D. Mechtcheriakova, N. Urtz, and T. Baumruker. 2003. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J. Biol. Chem. 278:47408-47415. - PubMed
-
- Clancy, C. J., M. H. Nguyen, R. Alandoerffer, S. Cheng, K. Iczkowski, M. Richardson, and J. R. Graybill. 2006. Cryptococcus neoformans var. grubii isolates recovered from persons with AIDS demonstrate a wide range of virulence during murine meningoencephalitis that correlates with the expression of certain virulence factors. Microbiology 152:2247-2255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

