Par1b/MARK2 phosphorylates kinesin-like motor protein GAKIN/KIF13B to regulate axon formation
- PMID: 20194617
- PMCID: PMC2863582
- DOI: 10.1128/MCB.01181-09
Par1b/MARK2 phosphorylates kinesin-like motor protein GAKIN/KIF13B to regulate axon formation
Abstract
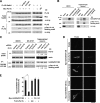
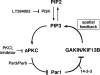
Here we report that Par1b/MARK2 regulates axon formation via phosphorylation of a kinesin superfamily protein GAKIN/KIF13B. Accumulating evidence indicated the importance of the evolutionarily conserved kinase Par1b in the regulation of cell polarity. Using hippocampal neurons in culture, it has been shown that Par1b regulates axon specification, but the underlying mechanism remains uncharacterized. We identify GAKIN/KIF13B as a novel Par1b-binding protein and reveal that GAKIN/KIF13B is a physiological substrate for Par1b, and the phosphorylation sites are conserved from Drosophila. In hippocampal neurons, GAKIN/KIF13B accumulates at the distal part of the microtubules in the tips of axons, but not of dendrites. Overexpression of GAKIN/KIF13B by itself can induce the formation of extra axons, which is inhibited by the coexpression of Par1b in a manner dependent on its kinase activity. In contrast, small interfering RNA (siRNA)-mediated knockdown of GAKIN/KIF13B severely retards neurite extension and promotes the axonless phenotype. The extra axon phenotype caused by Par1b siRNA is suppressed by cointroduction of GAKIN/KIF13B siRNA, thus placing the GAKIN/KIF13B function downstream of Par1b. We also find that GAKIN/KIF13B acts downstream of the phosphatidylinositol 3-kinase (PI3K) signaling via Par1b phosphorylation. These results reveal that GAKIN/KIF13B is a key intermediate linking Par1b to the regulation of axon formation.
Figures






Similar articles
-
Transport of PIP3 by GAKIN, a kinesin-3 family protein, regulates neuronal cell polarity.J Cell Biol. 2006 Jul 31;174(3):425-36. doi: 10.1083/jcb.200604031. Epub 2006 Jul 24. J Cell Biol. 2006. PMID: 16864656 Free PMC article.
-
Kinesin Regulation in the Proximal Axon is Essential for Dendrite-selective Transport.Mol Biol Cell. 2024 Jun 1;35(6):ar81. doi: 10.1091/mbc.E23-11-0457. Epub 2024 Apr 10. Mol Biol Cell. 2024. PMID: 38598291 Free PMC article.
-
Polarity-regulating kinase partitioning-defective 1/microtubule affinity-regulating kinase 2 negatively regulates development of dendrites on hippocampal neurons.J Neurosci. 2007 Nov 28;27(48):13098-107. doi: 10.1523/JNEUROSCI.3986-07.2007. J Neurosci. 2007. PMID: 18045904 Free PMC article.
-
Molecular motors and mechanisms of directional transport in neurons.Nat Rev Neurosci. 2005 Mar;6(3):201-14. doi: 10.1038/nrn1624. Nat Rev Neurosci. 2005. PMID: 15711600 Review.
-
One axon, many kinesins: What's the logic?J Neurocytol. 2000 Nov-Dec;29(11-12):799-818. doi: 10.1023/a:1010943424272. J Neurocytol. 2000. PMID: 11466472 Review.
Cited by
-
The FOXP2-Driven Network in Developmental Disorders and Neurodegeneration.Front Cell Neurosci. 2017 Jul 26;11:212. doi: 10.3389/fncel.2017.00212. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28798667 Free PMC article.
-
Smart motors and cargo steering drive kinesin-mediated selective transport.Mol Cell Neurosci. 2020 Mar;103:103464. doi: 10.1016/j.mcn.2019.103464. Epub 2020 Jan 20. Mol Cell Neurosci. 2020. PMID: 31972342 Free PMC article. Review.
-
Protein kinase A rescues microtubule affinity-regulating kinase 2-induced microtubule instability and neurite disruption by phosphorylating serine 409.J Biol Chem. 2015 Jan 30;290(5):3149-60. doi: 10.1074/jbc.M114.629873. Epub 2014 Dec 15. J Biol Chem. 2015. PMID: 25512381 Free PMC article.
-
Motor protein KIF13B orchestrates hepatic metabolism to prevent metabolic dysfunction-associated fatty liver disease.Mil Med Res. 2025 Mar 4;12(1):11. doi: 10.1186/s40779-025-00594-3. Mil Med Res. 2025. PMID: 40038775 Free PMC article.
-
The Plus End-Directed Microtubule (Kinesin-3 Family) Motor Protein KIF13B Is Associated with the Photoreceptor Synaptic Ribbon Complex.Int J Mol Sci. 2025 Jun 24;26(13):6044. doi: 10.3390/ijms26136044. Int J Mol Sci. 2025. PMID: 40649824 Free PMC article.
References
-
- Akhmanova, A., C. C. Hoogenraad, K. Drabek, T. Stepanova, B. Dortland, T. Verkerk, W. Vermeulen, B. M. Burgering, C. I. De Zeeuw, F. Grosveld, and N. Galjart. 2001. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell 104:923-935. - PubMed
-
- Arimura, N., and K. Kaibuchi. 2007. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 8:194-205. - PubMed
-
- Asaba, N., T. Hanada, A. Takeuchi, and A. H. Chisti. 2003. Direct interaction with a kinesin-related motor mediates transport of mammalian discs large tumor suppressor homologue in epithelial cells. J. Biol. Chem. 278:8395-8400. - PubMed
-
- Benton, R., and D. S. Johnston. 2003. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 115:691-704. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
