Molecular basis for lysine specificity in the yeast ubiquitin-conjugating enzyme Cdc34
- PMID: 20194622
- PMCID: PMC2863694
- DOI: 10.1128/MCB.01094-09
Molecular basis for lysine specificity in the yeast ubiquitin-conjugating enzyme Cdc34
Abstract
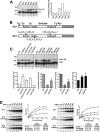
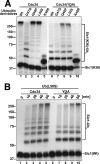
Ubiquitin (Ub)-conjugating enzymes (E2s) and ubiquitin ligases (E3s) catalyze the attachment of Ub to lysine residues in substrates and Ub during monoubiquitination and polyubiquitination. Lysine selection is important for the generation of diverse substrate-Ub structures, which provides versatility to this pathway in the targeting of proteins to different fates. The mechanisms of lysine selection remain poorly understood, with previous studies suggesting that the ubiquitination site(s) is selected by the E2/E3-mediated positioning of a lysine(s) toward the E2/E3 active site. By studying the polyubiquitination of Sic1 by the E2 protein Cdc34 and the RING E3 Skp1/Cul1/F-box (SCF) protein, we now demonstrate that in addition to E2/E3-mediated positioning, proximal amino acids surrounding the lysine residues in Sic1 and Ub are critical for ubiquitination. This mechanism is linked to key residues composing the catalytic core of Cdc34 and independent of SCF. Changes to these core residues altered the lysine preference of Cdc34 and specified whether this enzyme monoubiquitinated or polyubiquitinated Sic1. These new findings indicate that compatibility between amino acids surrounding acceptor lysine residues and key amino acids in the catalytic core of ubiquitin-conjugating enzymes is an important mechanism for lysine selection during ubiquitination.
Figures





References
-
- Banerjee, A., L. Gregori, Y. Xu, and V. Chau. 1993. The bacterially expressed yeast cdc34 gene product can undergo autoubiquitination to form a multiubiquitin chain-linked protein. J. Biol. Chem. 268:5668-5675. - PubMed
-
- Bernier-Villamor, V., D. A. Sampson, M. J. Matunis, and C. D. Lima. 2002. Structural basis for E2-mediated SUMO conjugation revealed by a complex between ubiquitin-conjugating enzyme Ubc9 and RanGAP1. Cell 108:345-356. - PubMed
-
- Block, K., T. Boyer, and P. Yew. 2001. Phosphorylation of the human ubiquitin-conjugating enzyme, Cdc34, by casein kinase 2. J. Biol. Chem. 276:41049-41058. - PubMed
-
- Boeke, J. D., J. Trueheart, G. Natsoulis, and G. R. Fink. 1987. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 154:164-175. - PubMed
-
- Deffenbaugh, A., K. Scaglione, L. Zhang, J. Moore, T. Buranda, L. Sklar, and D. Skowyra. 2003. Release of ubiquitin-charged Cdc34-S-Ub from the RING domain is essential for ubiquitination of the SCFcdc4-bound substrate SIC1. Cell 114:611-622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
