Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema
- PMID: 20194630
- PMCID: PMC2839150
- DOI: 10.1084/jem.20091513
Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema
Abstract
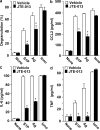
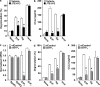
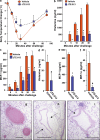
Systemic exacerbation of allergic responses, in which mast cells play a critical role, results in life-threatening anaphylactic shock. Sphingosine-1-phosphate (S1P), a ligand for a family of G protein-coupled receptors, is a new addition to the repertoire of bioactive lipids secreted by activated mast cells. Yet little is known of its role in human mast cell functions and in anaphylaxis. We show that S1P(2) receptors play a critical role in regulating human mast cell functions, including degranulation and cytokine and chemokine release. Immunoglobulin E-triggered anaphylactic responses, including elevation of circulating histamine and associated pulmonary edema in mice, were significantly attenuated by the S1P(2) antagonist JTE-013 and in S1P(2)-deficient mice, in contrast to anaphylaxis induced by administration of histamine or platelet-activating factor. Hence, S1P and S1P(2) on mast cells are determinants of systemic anaphylaxis and associated pulmonary edema and might be beneficial targets for anaphylaxis attenuation and prophylaxis.
Figures
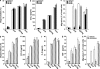
Similar articles
-
S1P₄ Regulates Passive Systemic Anaphylaxis in Mice but Is Dispensable for Canonical IgE-Mediated Responses in Mast Cells.Int J Mol Sci. 2018 Apr 25;19(5):1279. doi: 10.3390/ijms19051279. Int J Mol Sci. 2018. PMID: 29693558 Free PMC article.
-
The sphingosine-1-phosphate/sphingosine-1-phosphate receptor 2 axis regulates early airway T-cell infiltration in murine mast cell-dependent acute allergic responses.J Allergy Clin Immunol. 2015 Apr;135(4):1008-1018.e1. doi: 10.1016/j.jaci.2014.10.044. Epub 2014 Dec 13. J Allergy Clin Immunol. 2015. PMID: 25512083 Free PMC article.
-
Interrogation of sphingosine-1-phosphate receptor 2 function in vivo reveals a prominent role in the recovery from IgE and IgG-mediated anaphylaxis with minimal effect on its onset.Immunol Lett. 2013 Feb;150(1-2):89-96. doi: 10.1016/j.imlet.2013.01.005. Epub 2013 Jan 18. Immunol Lett. 2013. PMID: 23337656 Free PMC article.
-
Sphingosine-1-phosphate in allergic responses, asthma and anaphylaxis.Pharmacol Ther. 2007 Sep;115(3):390-9. doi: 10.1016/j.pharmthera.2007.05.011. Epub 2007 Jun 30. Pharmacol Ther. 2007. PMID: 17669501 Free PMC article. Review.
-
Unraveling the complexities of sphingosine-1-phosphate function: the mast cell model.Prostaglandins Other Lipid Mediat. 2008 Jun;86(1-4):1-11. doi: 10.1016/j.prostaglandins.2008.02.005. Epub 2008 Mar 4. Prostaglandins Other Lipid Mediat. 2008. PMID: 18403224 Free PMC article. Review.
Cited by
-
S1P control of endothelial integrity.Curr Top Microbiol Immunol. 2014;378:85-105. doi: 10.1007/978-3-319-05879-5_4. Curr Top Microbiol Immunol. 2014. PMID: 24728594 Free PMC article. Review.
-
Regulation of mast cell responses in health and disease.Crit Rev Immunol. 2011;31(6):475-529. doi: 10.1615/critrevimmunol.v31.i6.30. Crit Rev Immunol. 2011. PMID: 22321108 Free PMC article. Review.
-
PhotoImmunoNanoTherapy reveals an anticancer role for sphingosine kinase 2 and dihydrosphingosine-1-phosphate.ACS Nano. 2013 Mar 26;7(3):2132-44. doi: 10.1021/nn304862b. Epub 2013 Feb 14. ACS Nano. 2013. PMID: 23373542 Free PMC article.
-
Regulation of Immune Cell Migration by Sphingosine-1-Phosphate.Cell Mol Biol (OMICS). 2015;61(2):121. Epub 2015 Dec 31. Cell Mol Biol (OMICS). 2015. PMID: 30294722 Free PMC article.
-
Lipocalin 2: A New Antimicrobial in Mast Cells.Int J Mol Sci. 2019 May 14;20(10):2380. doi: 10.3390/ijms20102380. Int J Mol Sci. 2019. PMID: 31091692 Free PMC article.
References
-
- Ammit A.J., Hastie A.T., Edsall L.C., Hoffman R.K., Amrani Y., Krymskaya V.P., Kane S.A., Peters S.P., Penn R.B., Spiegel S., Panettieri R.A., Jr 2001. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB J. 15:1212–1214 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

