Integration of contractile forces during tissue invagination
- PMID: 20194639
- PMCID: PMC2835944
- DOI: 10.1083/jcb.200910099
Integration of contractile forces during tissue invagination
Abstract
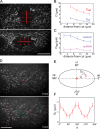
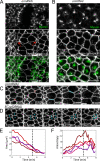
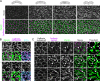
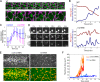
Contractile forces generated by the actomyosin cytoskeleton within individual cells collectively generate tissue-level force during epithelial morphogenesis. During Drosophila mesoderm invagination, pulsed actomyosin meshwork contractions and a ratchet-like stabilization of cell shape drive apical constriction. Here, we investigate how contractile forces are integrated across the tissue. Reducing adherens junction (AJ) levels or ablating actomyosin meshworks causes tissue-wide epithelial tears, which release tension that is predominantly oriented along the anterior-posterior (a-p) embryonic axis. Epithelial tears allow cells normally elongated along the a-p axis to constrict isotropically, which suggests that apical constriction generates anisotropic epithelial tension that feeds back to control cell shape. Epithelial tension requires the transcription factor Twist, which stabilizes apical myosin II, promoting the formation of a supracellular actomyosin meshwork in which radial actomyosin fibers are joined end-to-end at spot AJs. Thus, pulsed actomyosin contractions require a supracellular, tensile meshwork to transmit cellular forces to the tissue level during morphogenesis.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

