Interaction of Candida albicans biofilms with antifungals: transcriptional response and binding of antifungals to beta-glucans
- PMID: 20194705
- PMCID: PMC2863626
- DOI: 10.1128/AAC.01638-09
Interaction of Candida albicans biofilms with antifungals: transcriptional response and binding of antifungals to beta-glucans
Abstract
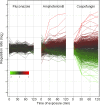
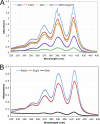
Candida albicans can form biofilms that exhibit elevated intrinsic resistance to various antifungal agents, in particular azoles and polyenes. The molecular mechanisms involved in the antifungal resistance of biofilms remain poorly understood. We have used transcript profiling to explore the early transcriptional responses of mature C. albicans biofilms exposed to various antifungal agents. Mature C. albicans biofilms grown under continuous flow were exposed for as long as 2 h to concentrations of fluconazole (FLU), amphotericin B (AMB), and caspofungin (CAS) that, while lethal for planktonic cells, were not lethal for biofilms. Interestingly, FLU-exposed biofilms showed no significant changes in gene expression over the course of the experiment. In AMB-exposed biofilms, 2.7% of the genes showed altered expression, while in CAS-exposed biofilms, 13.0% of the genes had their expression modified. In particular, exposure to CAS resulted in the upregulation of hypha-specific genes known to play a role in biofilm formation, such as ALS3 and HWP1. There was little overlap between AMB- or CAS-responsive genes in biofilms and those that have been identified as AMB, FLU, or CAS responsive in C. albicans planktonic cultures. These results suggested that the resistance of C. albicans biofilms to azoles or polyenes was due not to the activation of specific mechanisms in response to exposure to these antifungals but rather to the intrinsic properties of the mature biofilms. In this regard, our study led us to observe that AMB physically bound C. albicans biofilms and beta-glucans, which have been proposed to be major constituents of the biofilm extracellular matrix and to prevent azoles from reaching biofilm cells. Thus, enhanced extracellular matrix or beta-glucan synthesis during biofilm growth might prevent antifungals, such as azoles and polyenes, from reaching biofilm cells, thus limiting their toxicity to these cells and the associated transcriptional responses.
Figures
Similar articles
-
Putative role of beta-1,3 glucans in Candida albicans biofilm resistance.Antimicrob Agents Chemother. 2007 Feb;51(2):510-20. doi: 10.1128/AAC.01056-06. Epub 2006 Nov 27. Antimicrob Agents Chemother. 2007. PMID: 17130296 Free PMC article.
-
Transcriptional response to fluconazole and amphotericin B in Candida albicans biofilms.Res Microbiol. 2010 May;161(4):284-92. doi: 10.1016/j.resmic.2010.02.004. Epub 2010 Feb 17. Res Microbiol. 2010. PMID: 20170727
-
Transcriptional regulation of drug-resistance genes in Candida albicans biofilms in response to antifungals.J Med Microbiol. 2011 Sep;60(Pt 9):1241-1247. doi: 10.1099/jmm.0.030692-0. Epub 2011 Apr 7. J Med Microbiol. 2011. PMID: 21474609
-
Recent insights into Candida albicans biofilm resistance mechanisms.Curr Genet. 2013 Nov;59(4):251-64. doi: 10.1007/s00294-013-0400-3. Epub 2013 Aug 25. Curr Genet. 2013. PMID: 23974350 Free PMC article. Review.
-
The effect of biomaterials and antifungals on biofilm formation by Candida species: a review.Eur J Clin Microbiol Infect Dis. 2012 Oct;31(10):2513-27. doi: 10.1007/s10096-012-1634-6. Epub 2012 May 12. Eur J Clin Microbiol Infect Dis. 2012. PMID: 22581304 Review.
Cited by
-
Genomic and Molecular Identification of Genes Contributing to the Caspofungin Paradoxical Effect in Aspergillus fumigatus.Microbiol Spectr. 2022 Oct 26;10(5):e0051922. doi: 10.1128/spectrum.00519-22. Epub 2022 Sep 12. Microbiol Spectr. 2022. PMID: 36094204 Free PMC article.
-
Gymnemic Acids Inhibit Adhesive Nanofibrillar Mediated Streptococcus gordonii-Candida albicans Mono-Species and Dual-Species Biofilms.Front Microbiol. 2019 Oct 11;10:2328. doi: 10.3389/fmicb.2019.02328. eCollection 2019. Front Microbiol. 2019. PMID: 31681200 Free PMC article.
-
Transcriptomic and Genomic Approaches for Unravelling Candida albicans Biofilm Formation and Drug Resistance-An Update.Genes (Basel). 2018 Nov 7;9(11):540. doi: 10.3390/genes9110540. Genes (Basel). 2018. PMID: 30405082 Free PMC article. Review.
-
Mechanisms of Candida biofilm drug resistance.Future Microbiol. 2013 Oct;8(10):1325-37. doi: 10.2217/fmb.13.101. Future Microbiol. 2013. PMID: 24059922 Free PMC article. Review.
-
Fluconazole impacts the extracellular matrix of fluconazole-susceptible and -resistant Candida albicans and Candida glabrata biofilms.J Oral Microbiol. 2018 Jun 4;10(1):1476644. doi: 10.1080/20002297.2018.1476644. eCollection 2018. J Oral Microbiol. 2018. PMID: 29887974 Free PMC article.
References
-
- Al-Fattani, M. A., and L. J. Douglas. 2006. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J. Med. Microbiol. 55:999-1008. - PubMed
-
- Anderson, G. G., and G. A. O'Toole. 2008. Innate and induced resistance mechanisms of bacterial biofilms. Curr. Top. Microbiol. Immunol. 322:85-105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

