CXC chemokine ligand (CXCL) 9 and CXCL10 are antagonistic costimulation molecules during the priming of alloreactive T cell effectors
- PMID: 20194716
- PMCID: PMC2885704
- DOI: 10.4049/jimmunol.0903831
CXC chemokine ligand (CXCL) 9 and CXCL10 are antagonistic costimulation molecules during the priming of alloreactive T cell effectors
Abstract
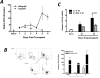
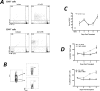
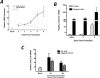
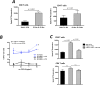
Donor Ag-reactive CD4 and CD8 T cell production of IFN-gamma is a principal effector mechanism promoting tissue injury during allograft rejection. The CXCR3-binding chemokines CXCL9 and CXCL10 recruit donor-reactive T cells to the allograft, but their role during the priming of donor-reactive T cells to effector function is unknown. Using a murine model of MHC-mismatched cardiac transplantation, we investigated the influence of CXCL9 and CXCL10 during donor-reactive T cell priming. In allograft recipient spleens, CXCL9 and CXCL10 were expressed as early as 24 h posttransplant and increased with similar kinetics, concurrently with CXCR3 expression on T cells. CXCL9, but not CXCL10, expression required NK cell production of IFN-gamma. The absence of CXCL9 in donor allografts, recipients, or both significantly decreased the frequency of donor-reactive CD8 T cells producing IFN-gamma and increased the frequency of donor-reactive CD8 T cells producing IL-17A. In contrast, the absence of CXCL10 increased the frequency of IFN-gamma-producing CD8 T cells in a CXCL9-dependent manner. These data provide novel evidence that donor-reactive CD8 T cells use the CXCR3 chemokine axis as a costimulation pathway during priming to allografts where CXCL9 promotes the development of IFN-gamma-producing CD8 T cells, and CXCL10 antagonizes this skewing.
Figures




Similar articles
-
Chemokine monokine induced by IFN-gamma/CXC chemokine ligand 9 stimulates T lymphocyte proliferation and effector cytokine production.J Immunol. 2004 Jun 15;172(12):7417-24. doi: 10.4049/jimmunol.172.12.7417. J Immunol. 2004. PMID: 15187119
-
Heterodimeric IL-15 delays tumor growth and promotes intratumoral CTL and dendritic cell accumulation by a cytokine network involving XCL1, IFN-γ, CXCL9 and CXCL10.J Immunother Cancer. 2020 May;8(1):e000599. doi: 10.1136/jitc-2020-000599. J Immunother Cancer. 2020. PMID: 32461349 Free PMC article.
-
NOD2 regulates CXCR3-dependent CD8+ T cell accumulation in intestinal tissues with acute injury.J Immunol. 2014 Apr 1;192(7):3409-18. doi: 10.4049/jimmunol.1302436. Epub 2014 Mar 3. J Immunol. 2014. PMID: 24591373 Free PMC article.
-
CXCR3 Ligands in Cancer and Autoimmunity, Chemoattraction of Effector T Cells, and Beyond.Front Immunol. 2020 May 29;11:976. doi: 10.3389/fimmu.2020.00976. eCollection 2020. Front Immunol. 2020. PMID: 32547545 Free PMC article. Review.
-
Hypersensitivity pneumonitis and alpha-chemokines.Clin Ter. 2017 Mar-Apr;168(2):e140-e145. doi: 10.7417/CT.2017.1996. Clin Ter. 2017. PMID: 28383627 Review.
Cited by
-
Combination of C-X-C motif chemokine 9 and C-X-C motif chemokine 10 antibodies with FTY720 prolongs the survival of cardiac retransplantation allografts in a mouse model.Exp Ther Med. 2015 Mar;9(3):1006-1012. doi: 10.3892/etm.2015.2204. Epub 2015 Jan 22. Exp Ther Med. 2015. PMID: 25667668 Free PMC article.
-
Characterization and Proteomic Analyses of Proinflammatory Cytokines in a Mouse Model of Liver Transplant Rejection.Oxid Med Cell Longev. 2022 Aug 12;2022:5188584. doi: 10.1155/2022/5188584. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35993024 Free PMC article.
-
Hepatocyte growth factor in dampening liver immune-mediated pathology in acute viral hepatitis without compromising antiviral activity.J Gastroenterol Hepatol. 2014 Apr;29(4):878-86. doi: 10.1111/jgh.12456. J Gastroenterol Hepatol. 2014. PMID: 24224701 Free PMC article.
-
A plasmatic score using a miRNA signature and CXCL-10 for accurate prediction and diagnosis of liver allograft rejection.Front Immunol. 2023 May 30;14:1196882. doi: 10.3389/fimmu.2023.1196882. eCollection 2023. Front Immunol. 2023. PMID: 37325660 Free PMC article.
-
Glycosaminoglycans Regulate CXCR3 Ligands at Distinct Levels: Protection against Processing by Dipeptidyl Peptidase IV/CD26 and Interference with Receptor Signaling.Int J Mol Sci. 2017 Jul 13;18(7):1513. doi: 10.3390/ijms18071513. Int J Mol Sci. 2017. PMID: 28703769 Free PMC article.
References
-
- Heeger PS. T-cell allorecognition and transplant rejection: a summary and update. Am J Transplant. 2003;3:525–533. - PubMed
-
- Hall BM, Dorsch SE. Cells mediating allograft rejection. Immunol Rev. 1984;77:31–59. - PubMed
-
- Hidalgo LG, Halloran PF. Role of IFN-gamma in allograft rejection. Crit Rev Immunol. 2002;22:317–349. - PubMed
-
- Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

