Cleavage of transaldolase by granzyme B causes the loss of enzymatic activity with retention of antigenicity for multiple sclerosis patients
- PMID: 20194725
- PMCID: PMC3117466
- DOI: 10.4049/jimmunol.0804174
Cleavage of transaldolase by granzyme B causes the loss of enzymatic activity with retention of antigenicity for multiple sclerosis patients
Abstract
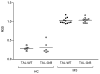
Multiple sclerosis (MS) is an autoimmune demyelinating disease of the CNS resulting from a progressive loss of oligodendrocytes. Transaldolase (TAL) is expressed at selectively high levels in oligodendrocytes of the brain, and postmortem sections show concurrent loss of myelin basic protein and TAL from sites of demyelination. Infiltrating CD8(+) CTLs are thought to play a key role in oligodendrocyte cell death. Cleavage by granzyme B (GrB) is predictive for autoantigenicity of self-proteins, thereby further implicating CTL-induced death in the initiation and propagation of autoimmunity. The precursor frequency and CTL activity of HLA-A2-restricted TAL 168-176-specific CD8(+) T cells is increased in MS patients. In this paper, we show that TAL, but not myelin basic protein, is specifically cleaved by human GrB. The recognition site of GrB that resulted in the cleavage of a dominant TAL fragment was mapped to a VVAD motif at aa residue 27 by N-terminal sequencing and confirmed by site-directed mutagenesis. The major C-terminal GrB cleavage product, residues 28-337, had no enzymatic activity but retained the antigenicity of full-length TAL, effectively stimulating the proliferation and CTL activity of PBMCs and of CD8(+) T cell lines from patients with MS. Sera of MS patients exhibited similar binding affinity to wild-type and GrB-cleaved TAL. Because GrB mediates the killing of target cells and cleavage by GrB is predictive of autoantigen status of self proteins, GrB-cleaved TAL-specific T cell-mediated cytotoxicity may contribute to the progressive destruction of oligodendrocytes in patients with MS.
Figures



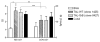
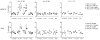
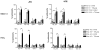
Similar articles
-
CD8+ T cell-mediated HLA-A*0201-restricted cytotoxicity to transaldolase peptide 168-176 in patients with multiple sclerosis.J Immunol. 2005 Dec 15;175(12):8365-78. doi: 10.4049/jimmunol.175.12.8365. J Immunol. 2005. PMID: 16339578
-
Oligodendrocyte-specific expression and autoantigenicity of transaldolase in multiple sclerosis.J Exp Med. 1994 Nov 1;180(5):1649-63. doi: 10.1084/jem.180.5.1649. J Exp Med. 1994. PMID: 7964452 Free PMC article.
-
Comparative analysis of antibody and cell-mediated autoimmunity to transaldolase and myelin basic protein in patients with multiple sclerosis.J Clin Invest. 1997 Mar 15;99(6):1238-50. doi: 10.1172/JCI119281. J Clin Invest. 1997. PMID: 9077532 Free PMC article.
-
Granzyme B cleavage of autoantigens in autoimmunity.Cell Death Differ. 2010 Apr;17(4):624-32. doi: 10.1038/cdd.2009.197. Epub 2010 Jan 15. Cell Death Differ. 2010. PMID: 20075942 Free PMC article. Review.
-
[Epitopes on myelin proteins recognized by autoantibodies present in multiple sclerosis patients].Postepy Hig Med Dosw (Online). 2004;58:472-82. Postepy Hig Med Dosw (Online). 2004. PMID: 15765008 Review. Polish.
Cited by
-
Granzyme B is elevated in autoimmune blistering diseases and cleaves key anchoring proteins of the dermal-epidermal junction.Sci Rep. 2018 Jun 26;8(1):9690. doi: 10.1038/s41598-018-28070-0. Sci Rep. 2018. PMID: 29946113 Free PMC article.
-
Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase.Trends Mol Med. 2011 Jul;17(7):395-403. doi: 10.1016/j.molmed.2011.01.014. Epub 2011 Mar 2. Trends Mol Med. 2011. PMID: 21376665 Free PMC article. Review.
-
An Autoantigen Profile of Human A549 Lung Cells Reveals Viral and Host Etiologic Molecular Attributes of Autoimmunity in COVID-19.bioRxiv [Preprint]. 2021 Feb 22:2021.02.21.432171. doi: 10.1101/2021.02.21.432171. bioRxiv. 2021. Update in: J Autoimmun. 2021 Jun;120:102644. doi: 10.1016/j.jaut.2021.102644. PMID: 33655248 Free PMC article. Updated. Preprint.
-
Granzyme B mediated function of Parvovirus B19-specific CD4(+) T cells.Clin Transl Immunology. 2015 Jul 3;4(7):e39. doi: 10.1038/cti.2015.13. eCollection 2015 Jul. Clin Transl Immunology. 2015. PMID: 26246896 Free PMC article.
-
Anti-proliferative and anti-tumour effects of lymphocyte-derived microparticles are neither species- nor tumour-type specific.J Extracell Vesicles. 2014 May 9;3. doi: 10.3402/jev.v3.23034. eCollection 2014. J Extracell Vesicles. 2014. PMID: 24834146 Free PMC article.
References
-
- Booss J, Esiri MM, Tourtellotte WW, Mason DY. Immunohistological analysis of T lymphocyte subsets in the central nervous system in chronic progressive multiple sclerosis. J Neurol Sci. 1983;62:219–232. - PubMed
-
- Hauser SL, Bhan AK, Gilles F, Kemp M, Kerr C, Weiner HL. Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions. Ann Neurol. 1986;19:578–587. - PubMed
-
- Traugott U, Scheinberg LC, Raine CS. On the presence of Ia-positive endothelial cells and astrocytes in multiple sclerosis lesions and its relevance to antigen presentation. J Neuroimmunol. 1985;8:1–14. - PubMed
-
- Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. [Review] Annu Rev Immunol. 1992;10:153–187. - PubMed
-
- Roder J, Hickey WF. Mouse models, immunology, multiple sclerosis and myelination. [news] Nat Genet. 1996;12:6–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

